Question
Question: How many of the following ethers cannot be prepared by Williamson's synthesis? CH3OCH2CH3, C6H5OCH3...
How many of the following ethers cannot be prepared by Williamson's synthesis?
CH3OCH2CH3, C6H5OCH3, C6H5OCH2CH3, (C6H5)2O, (CH3)3COCH3, (CH3)3COCH2CH3, (CH3)3COC(CH3)3, (C2H5)2O, C6H5CH2OC2H5.
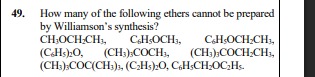
2
Solution
Williamson's synthesis is a method for preparing ethers by the reaction of an alkoxide with a primary alkyl halide. The general reaction is:
R-O⁻Na⁺ + R'-X → R-O-R' + NaX
Key considerations for Williamson's synthesis:
- Alkyl Halide (R'-X): It should ideally be a primary (1°) alkyl halide or methyl halide. Secondary (2°) and especially tertiary (3°) alkyl halides tend to undergo elimination (E2) reactions with strong bases like alkoxides, yielding alkenes instead of ethers.
- Alkoxide (R-O⁻): The alkoxide can be primary, secondary, or tertiary.
- Aryl/Vinyl Halides: Aryl halides (e.g., C6H5-X) and vinyl halides (e.g., CH2=CH-X) do not undergo SN2 reactions due to the partial double bond character of the C-X bond and steric hindrance, hence cannot be used as the R'-X component.
Let's analyze each given ether:
-
CH3OCH2CH3 (Methyl ethyl ether):
- Can be prepared from CH3CH2Br (primary) + CH3ONa. (Possible)
-
C6H5OCH3 (Anisole):
- Can be prepared from CH3Br (primary) + C6H5ONa (sodium phenoxide).
- Cannot be prepared from C6H5Br (aryl halide) + CH3ONa. (Possible by the first route)
-
C6H5OCH2CH3 (Phenetole):
- Can be prepared from CH3CH2Br (primary) + C6H5ONa (sodium phenoxide).
- Cannot be prepared from C6H5Br (aryl halide) + CH3CH2ONa. (Possible by the first route)
-
(C6H5)2O (Diphenyl ether):
- To form this, one would need C6H5Br (aryl halide) + C6H5ONa. Aryl halides do not undergo SN2 reactions required for Williamson's synthesis.
- Cannot be prepared.
-
(CH3)3COCH3 (tert-Butyl methyl ether):
- Can be prepared from CH3Br (primary) + (CH3)3CONa (sodium tert-butoxide).
- Cannot be prepared from (CH3)3CBr (tertiary alkyl halide) + CH3ONa, as this would lead to elimination. (Possible by the first route)
-
(CH3)3COCH2CH3 (tert-Butyl ethyl ether):
- Can be prepared from CH3CH2Br (primary) + (CH3)3CONa (sodium tert-butoxide).
- Cannot be prepared from (CH3)3CBr (tertiary alkyl halide) + CH3CH2ONa. (Possible by the first route)
-
(CH3)3COC(CH3)3 (Di-tert-butyl ether):
- To form this, one would need (CH3)3CBr (tertiary alkyl halide) + (CH3)3CONa (sodium tert-butoxide). A tertiary alkyl halide with a strong base/nucleophile will predominantly undergo E2 elimination, forming isobutylene, rather than SN2 substitution.
- Cannot be prepared.
-
(C2H5)2O (Diethyl ether):
- Can be prepared from C2H5Br (primary) + C2H5ONa (sodium ethoxide). (Possible)
-
C6H5CH2OC2H5 (Benzyl ethyl ether):
- Can be prepared from C2H5Br (primary) + C6H5CH2ONa (sodium benzyl alkoxide).
- Alternatively, can be prepared from C6H5CH2Br (primary/benzylic halide) + C2H5ONa (sodium ethoxide). Benzylic halides are good SN2 substrates. (Possible)
The ethers that cannot be prepared by Williamson's synthesis are:
- (C6H5)2O
- (CH3)3COC(CH3)3
There are 2 such ethers.
The final answer is 2
Explanation of the solution: Williamson's synthesis requires a primary alkyl halide and an alkoxide. Aryl halides and tertiary alkyl halides cannot be used as the halide component.
- (C6H5)2O: Requires an aryl halide (C6H5Br), which is unreactive in SN2.
- (CH3)3COC(CH3)3: Requires a tertiary alkyl halide ((CH3)3CBr), which undergoes elimination (E2) predominantly with strong alkoxide bases. All other listed ethers can be prepared by carefully choosing the primary alkyl halide and the appropriate alkoxide.
