Question
Question: If the electrode potential for a half-cell reaction at pH = 9, standard atmospheric pressure of oxyg...
If the electrode potential for a half-cell reaction at pH = 9, standard atmospheric pressure of oxygen 1 bar and 298K is given as x × 10⁻¹ volts. Then the value of x is _____. [Nearest integer]
2H+(aq)+21O2(g)+2e−→H2O(l);Eo=1.23V
(R = 8.314 J mol⁻¹ K⁻¹)
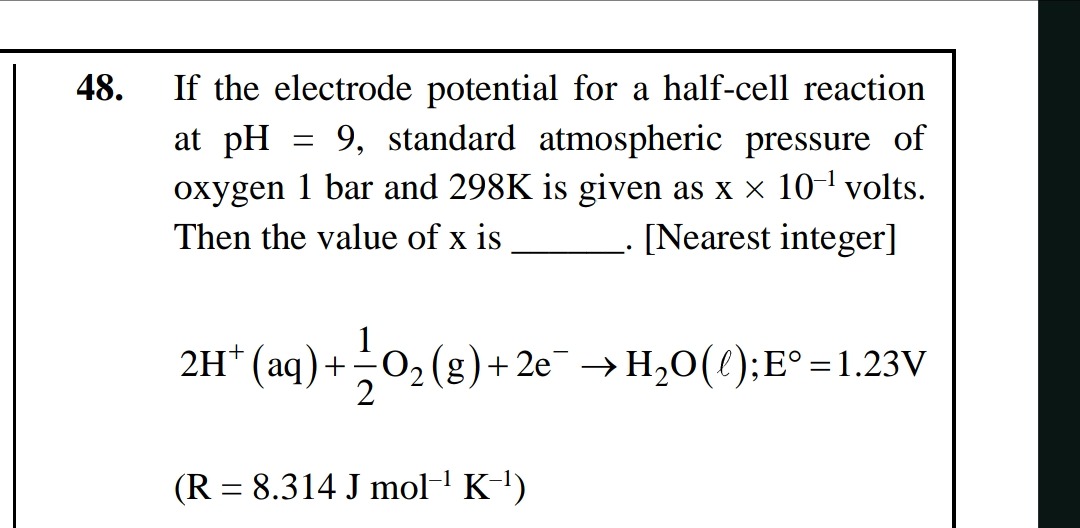
7
Solution
The problem asks us to calculate the electrode potential for a given half-cell reaction at specific conditions and then find the value of 'x'.
The given half-cell reaction is: 2H+(aq)+21O2(g)+2e−→H2O(l)
Given data: Standard electrode potential, Eo=1.23V Temperature, T=298K pH = 9 Standard atmospheric pressure of oxygen, PO2=1 bar Number of electrons transferred, n=2 (from the balanced half-reaction)
First, determine the hydrogen ion concentration [H+] from the pH: pH=−log10[H+] 9=−log10[H+] [H+]=10−9 M
Next, write the expression for the reaction quotient (Q) for the given reaction: Q=Activity of reactantsActivity of products For pure liquid water, its activity is 1. For gases, activity is approximated by partial pressure in bars. For aqueous species, activity is approximated by molar concentration. Q=[H+]2⋅(PO2)1/21
Substitute the values of [H+] and PO2 into the Q expression: Q=(10−9)2⋅(1)1/21 Q=10−18⋅11 Q=1018
Now, use the Nernst equation to calculate the electrode potential (E) at 298K: E=Eo−n0.0591log10Q
Substitute the known values into the Nernst equation: E=1.23−20.0591log10(1018) E=1.23−20.0591×18 E=1.23−0.0591×9 E=1.23−0.5319 E=0.6981 V
The problem states that the electrode potential is x×10−1 volts. So, x×10−1=0.6981 x=10−10.6981 x=0.6981×10 x=6.981
The question asks for the nearest integer value of x. The nearest integer to 6.981 is 7.
The final answer is 7.
Explanation of the solution:
- Determine [H+] from the given pH.
- Write the expression for the reaction quotient Q based on the given half-cell reaction.
- Calculate the value of Q using the calculated [H+] and given PO2.
- Apply the Nernst equation (E=Eo−n0.0591log10Q) to find the electrode potential E.
- Equate the calculated E to x×10−1 and solve for x.
- Round x to the nearest integer.
