Question
Question: The ratio of maximum to minimum wavelength in Balmer series of hydrogen atom is ..........
The ratio of maximum to minimum wavelength in Balmer series of hydrogen atom is .......
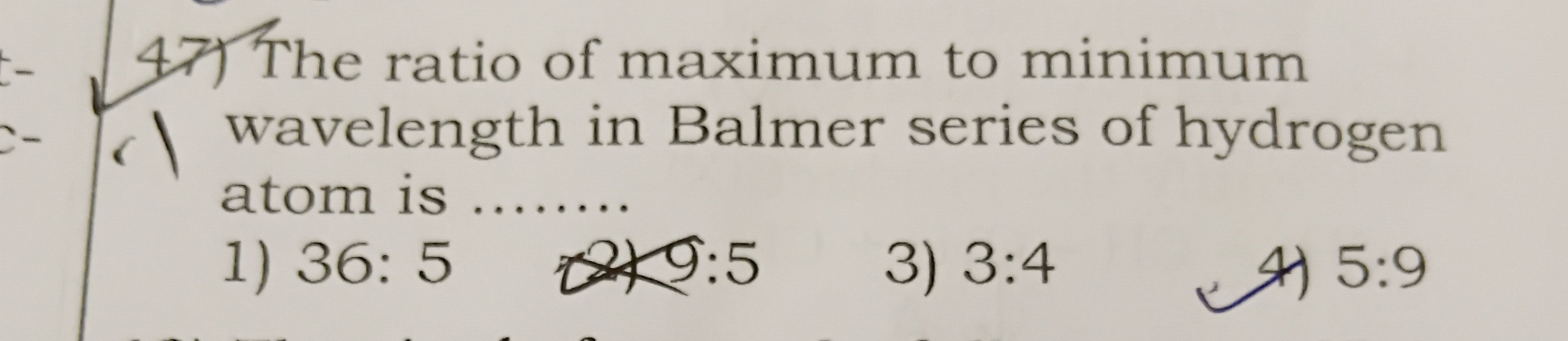
A
36: 5
B
9:5
C
3:4
D
5:9
Answer
9:5
Explanation
Solution
For the Balmer series, the wavelength is given by
λ1=R(221−n21)- The shortest wavelength (minimum wavelength, λmin) occurs for n=∞: λmin1=R(41−0)=4R.
- The longest wavelength (maximum wavelength, λmax) occurs for n=3: λmax1=R(41−91)=R(369−4)=365R.
Thus,
λmin=R4,λmax=5R36.The ratio of maximum to minimum wavelength is:
λminλmax=R45R36=536×4R×R1=2036=59.