Question
Question: The figure shows a sample of H-atoms having electron revolving in higher orbit 'n'. If this electron...
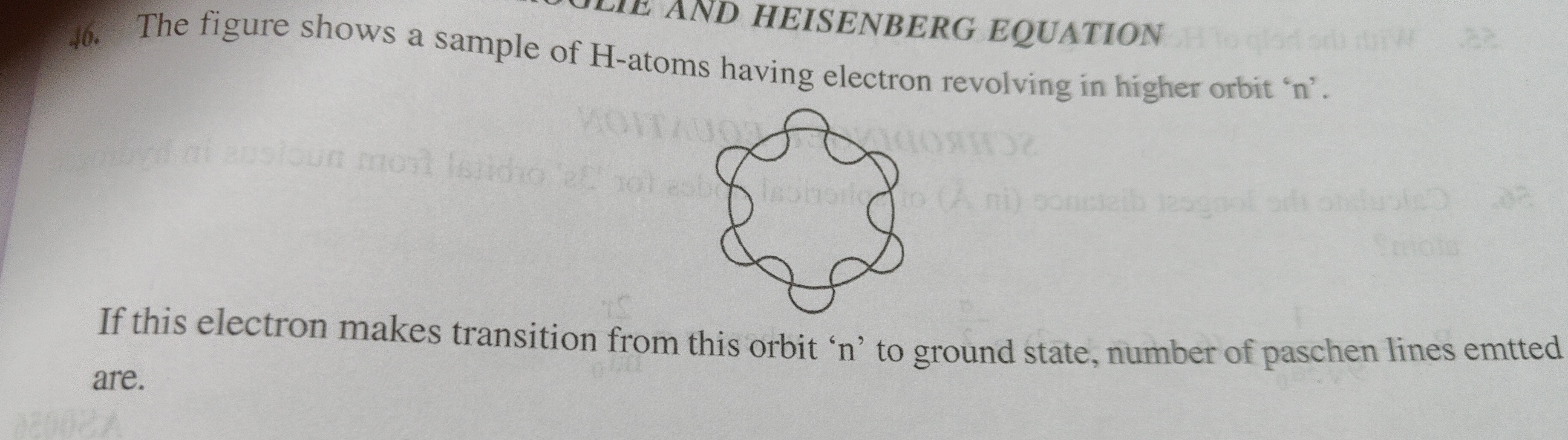
The figure shows a sample of H-atoms having electron revolving in higher orbit 'n'. If this electron makes transition from this orbit 'n' to ground state, number of paschen lines emtted are.
Answer
n-3
Explanation
Solution
The Paschen series corresponds to transitions to the n=3 energy level. For Paschen lines to be emitted from an initial orbit 'n', the electron must transition from an orbit k to n=3, where 3<k≤n. The possible initial orbits are 4,5,…,n. The number of such orbits is n−4+1=n−3. This is valid for n≥4.
