Question
Question: How many of the given reagents can be used to convert alcohol into alkyl halide? I. NaBr II. NaCl II...
How many of the given reagents can be used to convert alcohol into alkyl halide? I. NaBr II. NaCl III. HBr IV. PCl₃ V. SOCl₂ VI. HCl (conc.)/ZnCl₂ (anhyd.) VII. NaI VIII. PCl₅
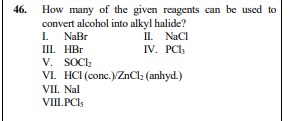
5
Solution
To convert an alcohol into an alkyl halide, the hydroxyl (-OH) group needs to be replaced by a halogen atom. This typically requires converting the poor leaving group (-OH) into a good leaving group (like H2O or a halide-phosphorus complex) and then allowing a halide ion to attack the carbon.
Let's analyze each given reagent:
-
I. NaBr: Sodium bromide is an ionic salt. The bromide ion (Br−) is a nucleophile, but it is not strong enough to displace the -OH group from an alcohol directly, as -OH is a poor leaving group. To use Br− effectively, the alcohol must first be protonated (e.g., by H2SO4) to convert -OH into a good leaving group (H2O+). Therefore, NaBr alone cannot convert alcohol to alkyl halide. It is typically used in conjunction with a strong acid to generate HBr in situ.
-
II. NaCl: Similar to NaBr, sodium chloride is an ionic salt. The chloride ion (Cl−) cannot displace -OH directly from an alcohol.
-
III. HBr: Hydrogen bromide is a strong acid and a source of Br−. It protonates the alcohol to form R-OH2+, which then loses water to form a carbocation (for 2° and 3° alcohols) or undergoes SN2 displacement (for 1° alcohols) by Br−. Thus, HBr can be used.
R-OH+HBr→R-Br+H2O
-
IV. PCl₃: Phosphorus trichloride is a commonly used reagent to convert alcohols to alkyl chlorides. It reacts to form phosphite acid as a byproduct.
3R-OH+PCl3→3R-Cl+H3PO3
-
V. SOCl₂: Thionyl chloride (often in the presence of pyridine) is an excellent reagent for converting alcohols to alkyl chlorides (Darzens reaction). The gaseous byproducts (SO2 and HCl) make purification of the alkyl halide easy.
R-OH+SOCl2pyridineR-Cl+SO2↑+HCl↑
-
VI. HCl (conc.)/ZnCl₂ (anhyd.): This combination is known as the Lucas reagent. It is effectively used to convert alcohols to alkyl chlorides. ZnCl2 acts as a Lewis acid, coordinating with the oxygen of the alcohol, making -OH a better leaving group.
R-OH+HClZnCl2R-Cl+H2O
-
VII. NaI: Similar to NaBr and NaCl, sodium iodide is an ionic salt and cannot convert alcohol to alkyl halide directly. It requires a strong acid (like H3PO4 or H2SO4) to generate HI in situ.
-
VIII. PCl₅: Phosphorus pentachloride is another effective reagent for converting alcohols to alkyl chlorides.
R-OH+PCl5→R-Cl+POCl3+HCl
Based on the analysis, the reagents that can be used to convert alcohol into alkyl halide are: III. HBr IV. PCl₃ V. SOCl₂ VI. HCl (conc.)/ZnCl₂ (anhyd.) VIII. PCl₅
There are 5 such reagents.
