Question
Question: Which of the following reagent would work best for the above conversion?...
Which of the following reagent would work best for the above conversion?
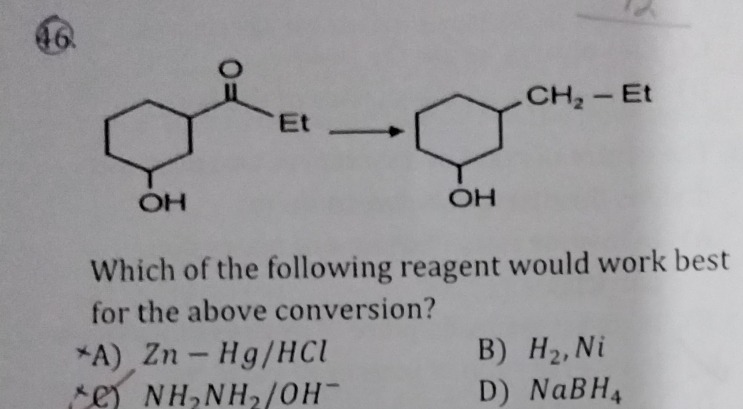
A
Zn-Hg/HCl
B
H₂, Ni
C
NH₂NH₂/OH⁻
D
NaBH₄
Answer
C
Explanation
Solution
The conversion involves reducing a ketone (-C(=O)-) to a methylene group (-CH₂-) while keeping a hydroxyl group (-OH) intact. This is a deoxygenation reaction. Clemmensen reduction (Zn-Hg/HCl) is acidic and would dehydrate the alcohol. H₂, Ni and NaBH₄ reduce ketones to alcohols, not alkanes. Wolff-Kishner reduction (NH₂NH₂/OH⁻) is basic and reduces ketones to alkanes, and alcohols are stable under basic conditions. Thus, Wolff-Kishner is the best choice.
