Question
Question: 46. Consider the following: ...
- Consider the following:
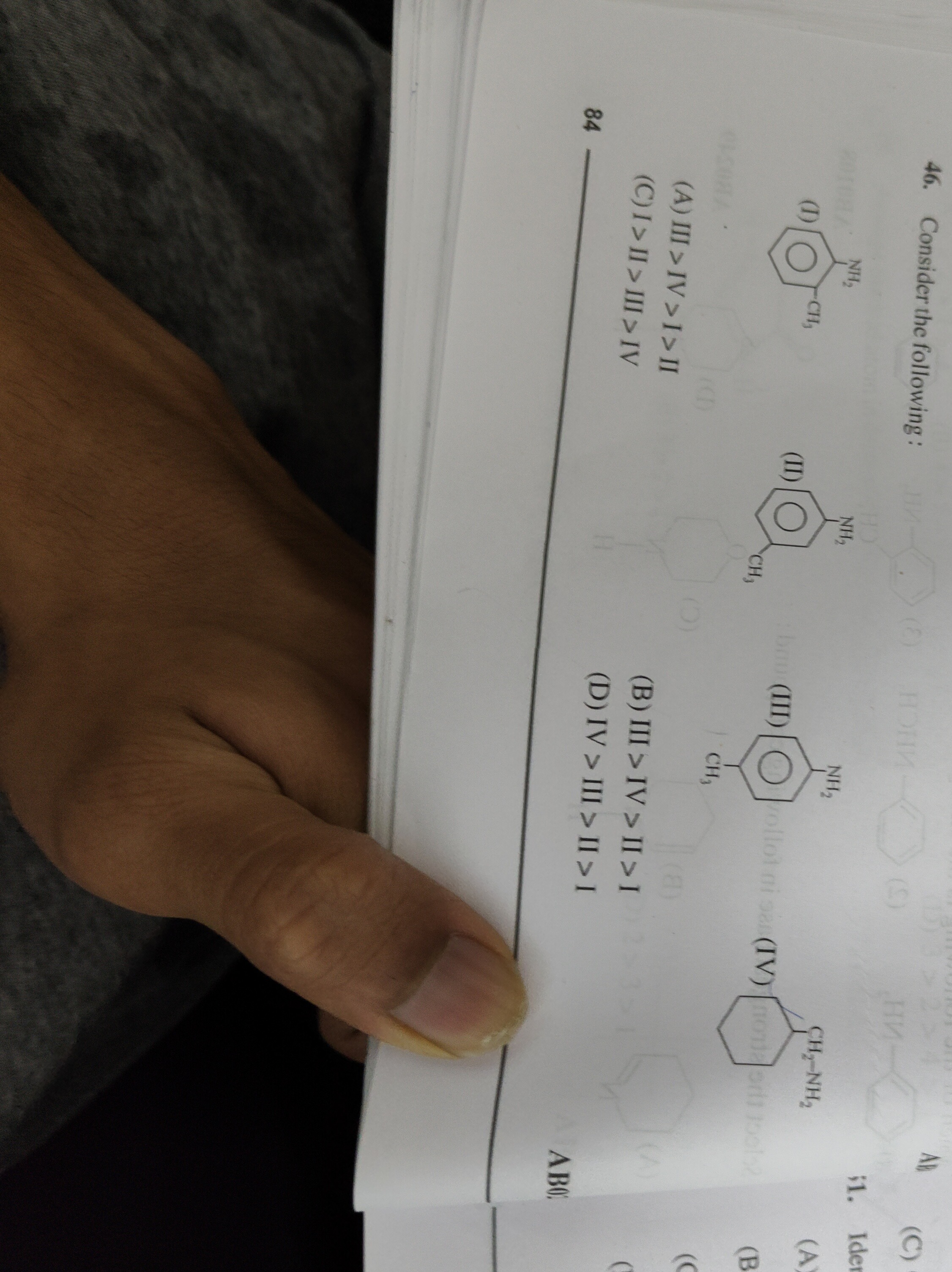
A
III > IV > I > II
B
I > II > III > IV
C
III > IV > II > I
D
IV > III > II > I
Answer
IV > III > II > I
Explanation
Solution
- Identify amine types: Compound IV is an aliphatic amine, while compounds I, II, and III are aromatic amines (anilines).
- Compare aliphatic vs. aromatic amines: Aliphatic amines are generally more basic than aromatic amines because the lone pair of electrons on the nitrogen atom in aliphatic amines is localized and readily available for protonation, whereas in aromatic amines, the lone pair is delocalized into the benzene ring through resonance, reducing its availability. Therefore, compound IV is the most basic.
- Analyze substituents on aromatic amines: Compounds I, II, and III are substituted anilines. The methyl group (-CH3) is an electron-donating group through inductive effect (+I) and hyperconjugation. Electron-donating groups increase the electron density on the nitrogen atom, thereby increasing basicity.
- Compare substituted anilines:
- Compound I is aniline (unsubstituted).
- Compound II is o-toluidine (methyl group at the ortho position).
- Compound III is p-toluidine (methyl group at the para position). The electron-donating effect of the methyl group makes II and III more basic than I. The para position (III) generally experiences a stronger activating effect than the ortho position (II) due to better transmission of electronic effects through the conjugated system. Furthermore, steric hindrance between the ortho methyl group and the amino group in II can reduce its basicity compared to III. Thus, the order of basicity among these three is III > II > I.
- Combine the orders: Since compound IV is significantly more basic than any of the aromatic amines, the overall order of basicity is IV > III > II > I.
