Question
Question: A peroxide in the absence of n. (Br = 80). A moth repellent has the composition 49% C, 2.7% H and 4...
A peroxide in the absence of n. (Br = 80).
A moth repellent has the composition 49% C, 2.7% H and 48.3% Cl. Its molecular weight gm. Determine its molecular formula.
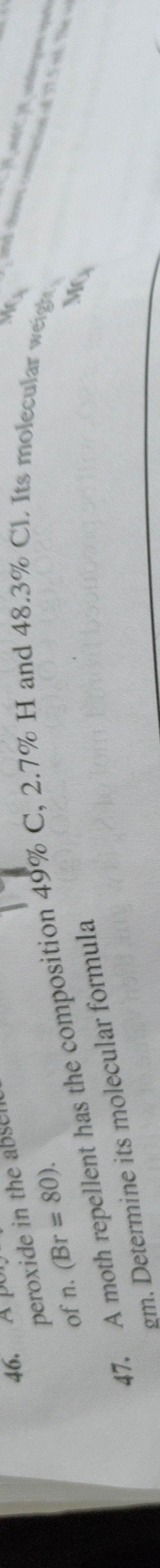
The molecular formula of the moth repellent is C6H4Cl2.
Solution
The question contains two parts, 46 and 47. Question 46 is incomplete and cannot be answered. We will solve question 47.
Solution for Question 47:
To determine the molecular formula, we follow these steps:
-
Calculate the moles of each element: Assume 100 g of the moth repellent.
-
Mass of Carbon (C) = 49 g Moles of C = 12 g/mol49 g=4.083 mol
-
Mass of Hydrogen (H) = 2.7 g Moles of H = 1 g/mol2.7 g=2.7 mol
-
Mass of Chlorine (Cl) = 48.3 g Moles of Cl = 35.5 g/mol48.3 g=1.361 mol
-
-
Determine the simplest whole-number ratio (Empirical Formula): Divide the number of moles of each element by the smallest number of moles (1.361 mol for Cl):
-
Ratio of C = 1.3614.083≈3
-
Ratio of H = 1.3612.7≈2
-
Ratio of Cl = 1.3611.361=1
The empirical formula is C3H2Cl.
-
-
Calculate the Empirical Formula Weight (EFW): EFW = (3 × Atomic mass of C) + (2 × Atomic mass of H) + (1 × Atomic mass of Cl)
EFW = (3 × 12) + (2 × 1) + (1 × 35.5) = 36 + 2 + 35.5 = 73.5 g/mol -
Determine the Molecular Formula: The question states "Its molecular weight gm." which is incomplete. For a complete problem, the molecular weight (MW) must be provided. Assuming a common molecular weight for a moth repellent that is a multiple of the empirical formula weight, such as 147 g/mol (which corresponds to p-dichlorobenzene, a common moth repellent):
-
Calculate the integer 'n' by which the empirical formula must be multiplied:
n=Empirical Formula WeightMolecular Weight
n=73.5 g/mol147 g/mol=2 -
Multiply the empirical formula by 'n' to get the molecular formula:
Molecular Formula = n×(C3H2Cl)=2×(C3H2Cl)=C6H4Cl2
-
The molecular formula of the moth repellent is C6H4Cl2.
Explanation of the solution:
- Convert given percentages to mass (assuming 100g sample) and then to moles for each element.
- Find the simplest whole-number ratio of moles to determine the empirical formula (C3H2Cl).
- Calculate the empirical formula weight (73.5 g/mol).
- Assume the molecular weight is 147 g/mol (as the question was incomplete).
- Divide the molecular weight by the empirical formula weight to find the integer 'n' (n=2).
- Multiply the empirical formula by 'n' to obtain the molecular formula (C6H4Cl2).
