Question
Question: When the following ketose exists in its D-configuration, the total number of stereoisomers in its fu...
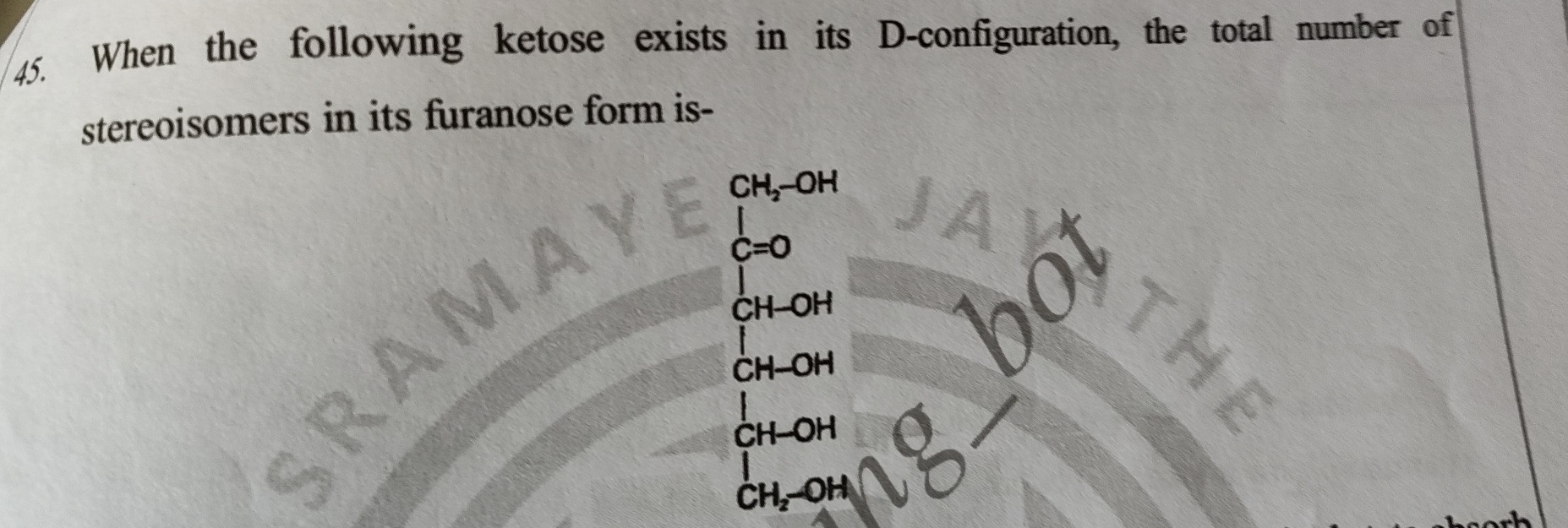
When the following ketose exists in its D-configuration, the total number of stereoisomers in its furanose form is-
8
Solution
The given structure is a ketohexose with the carbonyl group at C2. The chiral centers are C3, C4, and C5. The question states that the ketose exists in its D-configuration, which means the configuration at the chiral carbon farthest from the carbonyl group (C5) is D. In the Fischer projection, the hydroxyl group on C5 is on the right for the D-configuration. The configurations at C3 and C4 can vary, leading to different D-ketohexoses.
A furanose form is a five-membered ring. In ketohexoses, the furanose ring is formed by the reaction of the ketone group at C2 with the hydroxyl group at C5. This forms a cyclic hemiketal. The ring consists of C2, C3, C4, C5, and the oxygen atom from the C5 hydroxyl group. C1 and C6 are substituents outside the ring.
In the cyclic furanose form, C2 becomes a new chiral center, called the anomeric carbon. The original chiral centers are C3, C4, and C5. Thus, the furanose form of a ketohexose has chiral centers at C2, C3, C4, and C5.
Since the original open-chain ketose has chiral centers at C3, C4, and C5, and the configurations at C3 and C4 can vary while maintaining the D-configuration at C5, there are 22=4 possible D-ketohexoses with this skeleton (based on the configurations at C3 and C4). Let's denote the configurations at C3 and C4 as R or S. With C5 in the D-configuration, the possible D-ketohexoses differ in the configurations at C3 and C4.
For each of these 4 D-ketohexoses, cyclization to form the furanose ring introduces a new chiral center at C2 (the anomeric carbon). The configuration at C2 can be either α or β. The configurations at C3, C4, and C5 are determined by the original open-chain structure.
So, for each of the 4 distinct D-ketohexoses, there are two anomers (α and β) in the furanose form. Therefore, the total number of stereoisomers in the furanose form derived from all possible D-ketohexoses with this skeleton is 4×2=8. These 8 stereoisomers are all diastereomers of each other. Since we are considering D-configuration, we are not including the L-enantiomers.
