Question
Question: The incorrect order of bond angle is :...
The incorrect order of bond angle is :
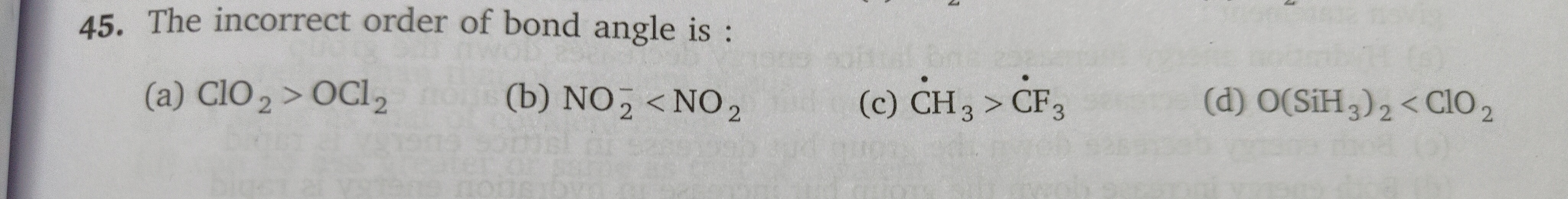
A
ClO2>OCl2
B
NO2−<NO2
C
C˙H3>C˙F3
D
O(SiH3)2<ClO2
Answer
O(SiH3)2<ClO2
Explanation
Solution
- ClO2: sp2 hybridized, 2 bonding pairs, 1 lone pair, 1 unpaired electron. Bond angle ~111°.
- OCl2: sp3 hybridized, 2 bonding pairs, 2 lone pairs. Bond angle < 109.5°, ~110.7°. Thus, ClO2>OCl2.
- NO2−: sp2 hybridized, 2 bonding pairs, 1 lone pair. Bond angle ~115°.
- NO2: sp2 hybridized, 2 bonding pairs, 1 lone pair, 1 unpaired electron. Unpaired electron is less repulsive than a lone pair. Bond angle ~134°. Thus, NO2−<NO2.
- C˙H3: sp2 hybridized, 3 bonding pairs, 1 unpaired electron. Bond angle ~119°.
- C˙F3: sp2 hybridized, 3 bonding pairs, 1 unpaired electron. Electronegativity of F and back-bonding reduce bond angle ~108-110°. Thus, C˙H3>C˙F3.
- O(SiH3)2: sp3 hybridized, 2 bonding pairs, 2 lone pairs. Back-bonding with Si increases bond angle to ~140-150°.
- Comparing O(SiH3)2 (~140-150°) and ClO2 (~111°), the order O(SiH3)2<ClO2 is incorrect.
