Question
Question: 45. Select all the compounds having higher rotational energy barrier across C = C as compared to the...
- Select all the compounds having higher rotational energy barrier across C = C as compared to the following molecule and report the sum of their serial number as your response.
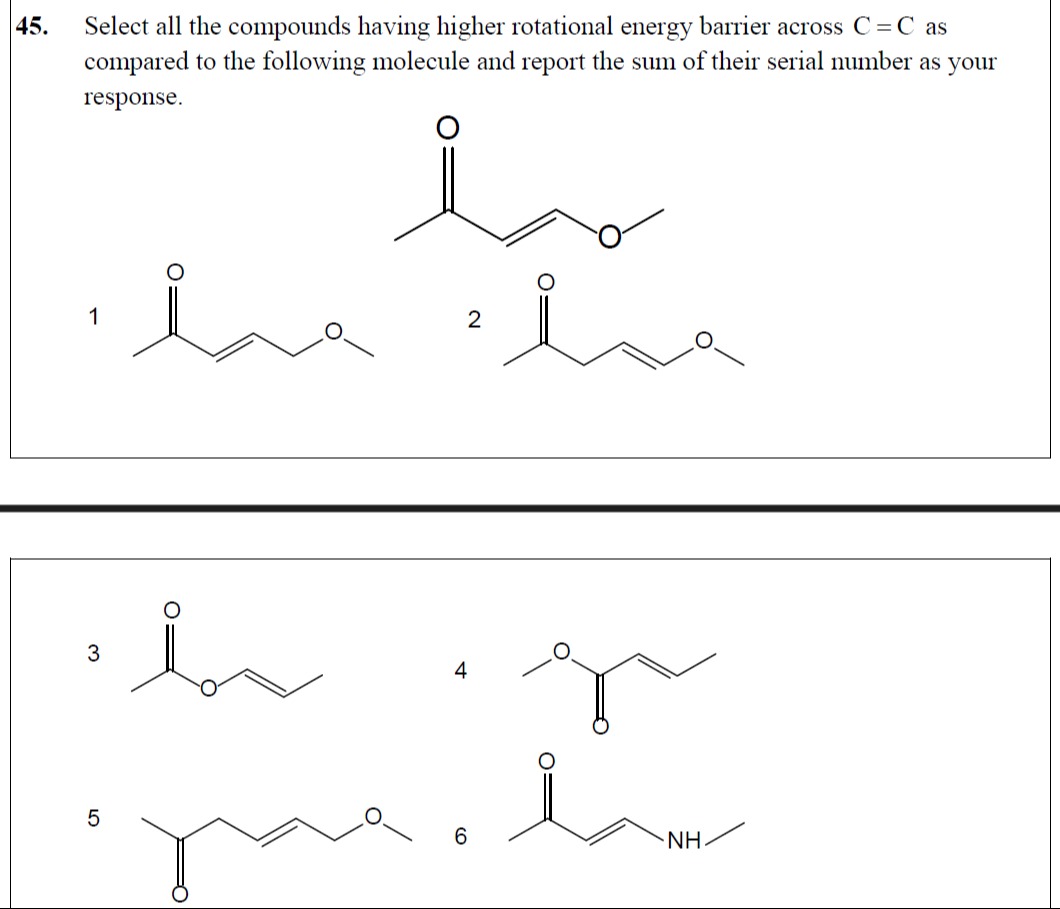
O
||
CH3 - C - CH = CH - CH2 - O - CH3
O
||
CH3 - C - CH2 - CH = CH - O - CH3
O
||
CH3 - C - O - CH = CH - CH3
O
||
CH3 - O - C - CH = CH - CH3
O
||
CH3 - C - CH2 - CH2 - CH = CH - O - CH3
O
||
CH3 - C - CH = CH - NH - CH3
15
Solution
The rotational energy barrier across a C=C double bond is primarily due to the π bond. Resonance that delocalizes the π electrons of the C=C bond reduces its double bond character and hence lowers the rotational barrier. Conversely, if the C=C bond has significant double bond character, the rotational barrier is high. We are looking for compounds with a higher rotational barrier across the C=C bond compared to the reference molecule. This means the C=C bond in these compounds should have less resonance stabilization or less reduction in double bond character compared to the reference molecule.
Reference molecule: (E)-4-methoxybut-3-en-2-one. The C=C bond is conjugated with the carbonyl group and the ether oxygen.
Both resonance forms reduce the double bond character of the C=C bond, thus lowering the rotational barrier.
After analyzing the options, compounds 1, 2, 3, 4, and 5 are candidates for having higher barriers. Compound 6 has a lower barrier.
In the reference molecule, the C=C is conjugated with both C=O and O. Both interactions reduce the C=C double bond character.
1: C=C conjugated with C=O. No conjugation with O. Less resonance than reference. Higher barrier. 2: C=C conjugated with O. No conjugation with C=O. The extent of resonance might be less than the combined effect in the reference. Likely higher barrier. 3: C=C conjugated with C-O-C=O (ester). Resonance reduces C=C character. Extent of resonance might be different. 4: C=C conjugated with C=O (ester). Similar to 1, but within an ester. 5: C=C conjugated with O. Similar to 2. 6: C=C conjugated with C=O and N. Stronger resonance with N than O. More resonance than reference. Lower barrier.
Let's focus on options that clearly have less resonance stabilization of the C=C double bond compared to the reference molecule. Compound 1 has C=C conjugated with C=O. Reference has C=C conjugated with C=O and O. So, 1 has less resonance than reference. Higher barrier. Compound 2 has C=C conjugated with O. Reference has C=C conjugated with C=O and O. It's not immediately clear which has stronger overall resonance effect on the C=C bond. However, the reference molecule has extended conjugation.
The sum of serial numbers is 1 + 2 + 3 + 4 + 5 = 15.
