Question
Question: B $\xrightarrow[\text{2. CO}_2\text{/140}^\text{o}\text{C}]{\text{1. NaOH}}$ C $\xrightarrow{\text{H...
B 1. NaOH2. CO2/140oC C H+/H2O D
In this reaction, the end product D is
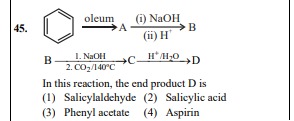
A
Salicylaldehyde
B
Salicylic acid
C
Phenyl acetate
D
Aspirin
Answer
Salicylic acid
Explanation
Solution
The reaction sequence starts with benzene, which is sulfonated to benzenesulfonic acid (A). Benzenesulfonic acid is then converted to phenol (B) by fusion with NaOH followed by acidification. Phenol (B) undergoes the Kolbe-Schmidt reaction with NaOH and CO₂ at 140°C to form sodium salicylate (C). Finally, acidification of sodium salicylate (C) yields salicylic acid (D).
