Question
Question: \(448{\text{ ml}}\) of a gaseous hydrocarbon (A) having \[{\text{C }}87.80\% \], \[{\text{H }}12.19\...
448 ml of a gaseous hydrocarbon (A) having C 87.80%, H 12.19% weighs 1.64 gms at STP. On hydrogenation it gives 2-methyl pentane. Treatment of (A) with acidic HgSO4 gives a new compound (B) M.F. C6H12O. Compound (A) does not react with ammoniacal AgNO3. Find out the structure of (A) and explain the reactions involved.
Solution
To solve this first calculate the molecular mass of the hydrocarbon. Then determine the molecular formula of the hydrocarbon from its empirical formula. We are given that hydrocarbon on hydrogenation gives an alkane. Thus, the hydrocarbon can be an alkene or alkyne.
Complete step-by-step solution:
We know that one mole of an ideal gas occupies a volume of 22.4 L=22.4×103 ml at STP i.e. standard temperature and pressure.
We are given that 448 ml of a gaseous hydrocarbon (A) weighs 1.64 gms at STP. Thus, 22.4×103 ml of gas at STP will weigh,
Weight of hydrocarbon=1.64 g×448 ml22.4×103 ml=82 g
Thus, one mole of hydrocarbon weighs 82 g at STP. Thus, the molecular weight of the hydrocarbon (A) is 82 g.
Determine the empirical formula of the hydrocarbon (A) as follows:
We are given that hydrocarbon (A) has C 87.80%, H 12.19%. Thus,
| Element(Given) | Percentage(Given) | Atomic mass(Known) | Relative ratio(Percentage/Atomic mass) | Relative number of atoms(Relative ratio/ Smallest relative ratio) | Simplest ratioRelative number of atoms multiplied by 3 |
|---|---|---|---|---|---|
| C | 87.80 | 12 | 7.31 | 1 | 3 |
| H | 12.19 | 1 | 12.19 | 1.66 | 5 |
We multiply the relative number of atoms by 3 to get a simplest whole number ratio.
Thus, the empirical formula of the hydrocarbon (A) is C3H5.
Determine the empirical formula mass as follows:
Empirical formula mass=(3×C)+(5×H)
Empirical formula mass=(3×12)+(5×1)
Empirical formula mass=41
Thus, the empirical formula mass of the hydrocarbon (A) is 41.
Determine the molecular formula of the hydrocarbon (A) as follows:
n=Empirical formula massMolecular mass
n=4182=2
Thus,
Molecular formula=Empirical formula×n
Molecular formula=C3H5×2
Molecular formula=C6H10×2
Thus, the molecular formula of the hydrocarbon (A) is C6H10.
We are given that hydrocarbon (A) does not react with ammoniacal AgNO3. Internal alkynes do not react with ammoniacal AgNO3. Thus, hydrocarbon (A) is an internal alkyne
We are given that hydrocarbon (A) on hydrogenation gives 2-methyl pentane which is an alkane. Alkynes on reaction with 2 moles of hydrogen gas give alkane. Thus, hydrocarbon (A) must be an internal alkyne with five carbon chains and methyl substituent at carbon number 4.
Thus, the structure of hydrocarbon (A) is as follows:
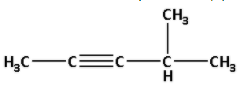
Thus, hydrocarbon (A) is 4-methyl pent-2-yne.
The reaction of hydrocarbon (A) with 2 moles of hydrogen gas is as follows:
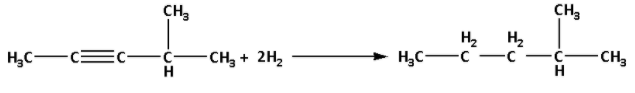
We are given that hydrocarbon (A) reacts with acidic HgSO4 and gives a new compound (B) having M.F. C6H12O.
Reaction of an alkyne with acidic HgSO4 gives an aldehyde or ketone. Thus, compound B can be an aldehyde or a ketone.
Note: Remember that terminal alkynes do not react with ammoniacal silver nitrate. Only internal alkynes react with ammoniacal silver nitrate. Terminal alkynes are alkynes having a triple bond at terminal position whereas internal alkynes are non-terminal alkynes.
