Question
Question: Which of the following cyclic carbocations is aromatic?...
Which of the following cyclic carbocations is aromatic?
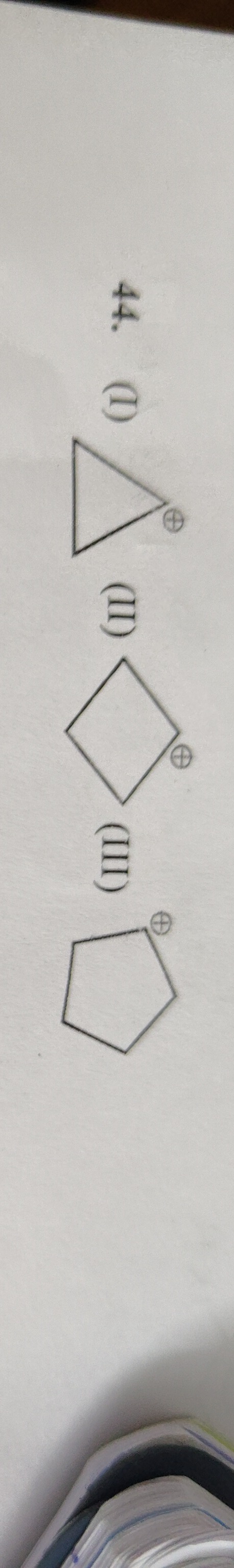
A
I
B
II
C
III
D
I and III
Answer
Structure (I) is aromatic.
Explanation
Solution
Aromaticity requires a cyclic, planar structure with a continuous π system and (4n+2) π electrons. (I) Cyclopropyl cation: It is planar and has 2 π electrons, satisfying Hückel's rule (n=0), thus it is aromatic. (II) Cyclobutyl cation: It is not planar and lacks a continuous π system, hence not aromatic. (III) Cyclopentyl cation: It is not planar and lacks a continuous π system, hence not aromatic. Therefore, only structure (I) is aromatic.
