Question
Question: If shortest wavelength of H-atom in Balmer series is xÅ then, (i) The shortest wave length in Lyman ...
If shortest wavelength of H-atom in Balmer series is xÅ then, (i) The shortest wave length in Lyman series is yxÅ. The value of 'y' is. (ii) The longest wave length in Paschen series is zxÅ. The value of 'z' is.
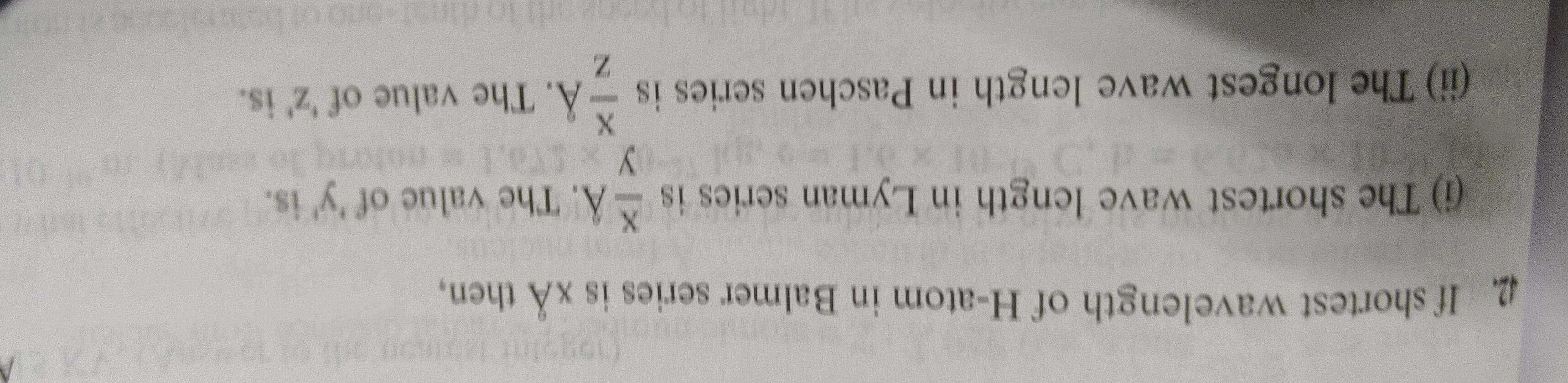
For (i): y = 4. For (ii): z = 7/36.
Solution
The Rydberg formula for the wavelength of spectral lines is: λ1=RZ2(nf21−ni21) For a Hydrogen atom, Z=1.
The shortest wavelength in the Balmer series corresponds to the transition from ni=∞ to nf=2. Given this shortest wavelength is x Å: x1=R(221−∞21)=R(41) This implies R=x4.
(i) The shortest wavelength in the Lyman series corresponds to the transition from ni=∞ to nf=1. λL1=R(121−∞21)=R Substituting the value of R: λL1=x4⟹λL=4x Given λL=yx, we find y=4.
(ii) The longest wavelength in the Paschen series corresponds to the transition from ni=4 to nf=3 (the smallest energy difference for nf=3). λP1=R(321−421)=R(91−161)=R(14416−9)=R(1447) Substituting the value of R: λP1=x4(1447)=144x28=36x7 Therefore, λP=736x. Given λP=zx, we have: 736x=zx⟹z=367
