Question
Question: A solution of 8 g of certain organic compound in 2 dm³ water develops osmotic pressure 0.6 atm at 30...
A solution of 8 g of certain organic compound in 2 dm³ water develops osmotic pressure 0.6 atm at 300 K. Calculate the molar mass of compound. [R = 0.082 atm dm³ K⁻¹ mol⁻¹]
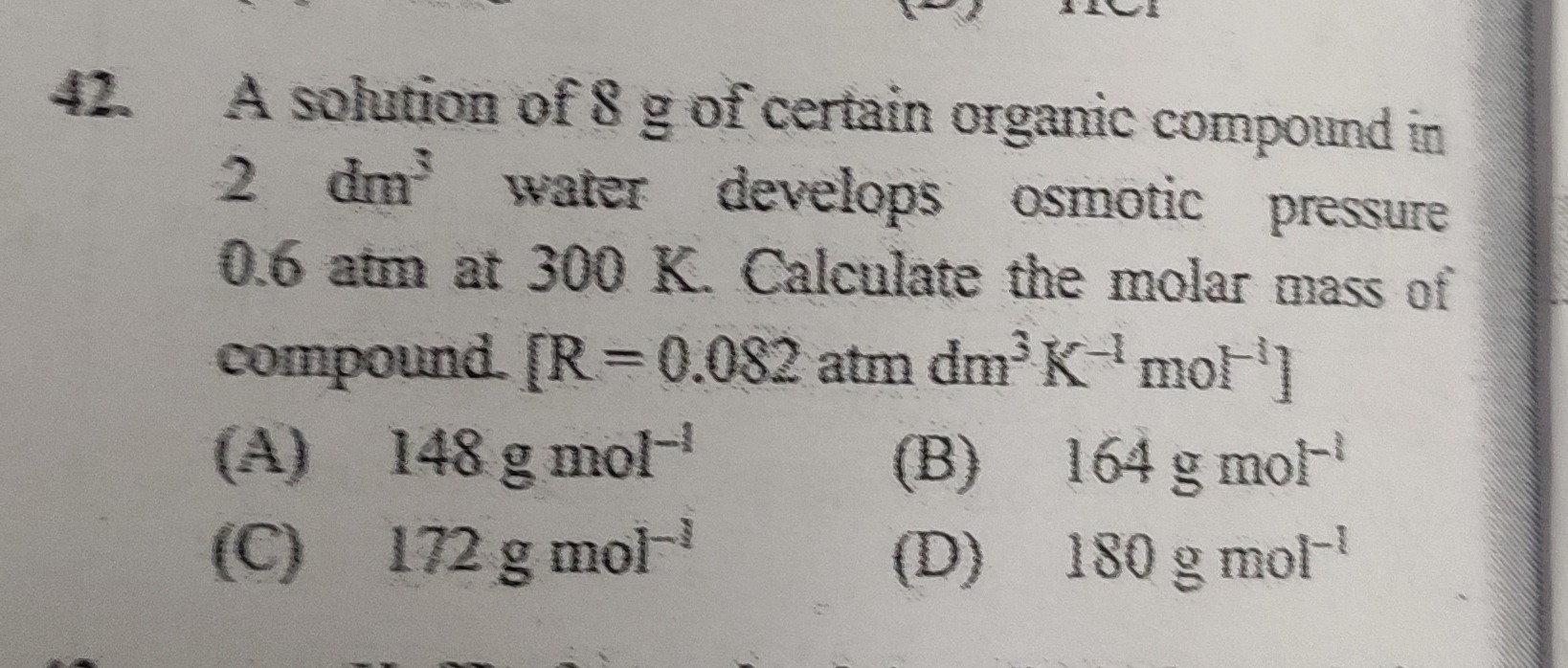
A
148 g mol⁻¹
B
164 g mol⁻¹
C
172 g mol⁻¹
D
180 g mol⁻¹
Answer
164 g mol⁻¹
Explanation
Solution
-
Calculate the number of moles of the compound:
Moles=molar massmass=M8 -
Determine the molarity of the solution:
Molarity,c=2(8/M)=M4 -
Use the osmotic pressure formula:
π=cRT
Substitute the values:
0.6=(M4)(0.082)(300) -
Solve for M:
4×0.082×300=98.4
So, 0.6=M98.4
⇒M=0.698.4=164g/mol
