Question
Question: H₂ and O₂ are kept in mass ratio 1 : 8 respectively at 6 atm. If small orifice is made, then moles o...
H₂ and O₂ are kept in mass ratio 1 : 8 respectively at 6 atm. If small orifice is made, then moles of H₂ with respect to O₂ coming out initially, is
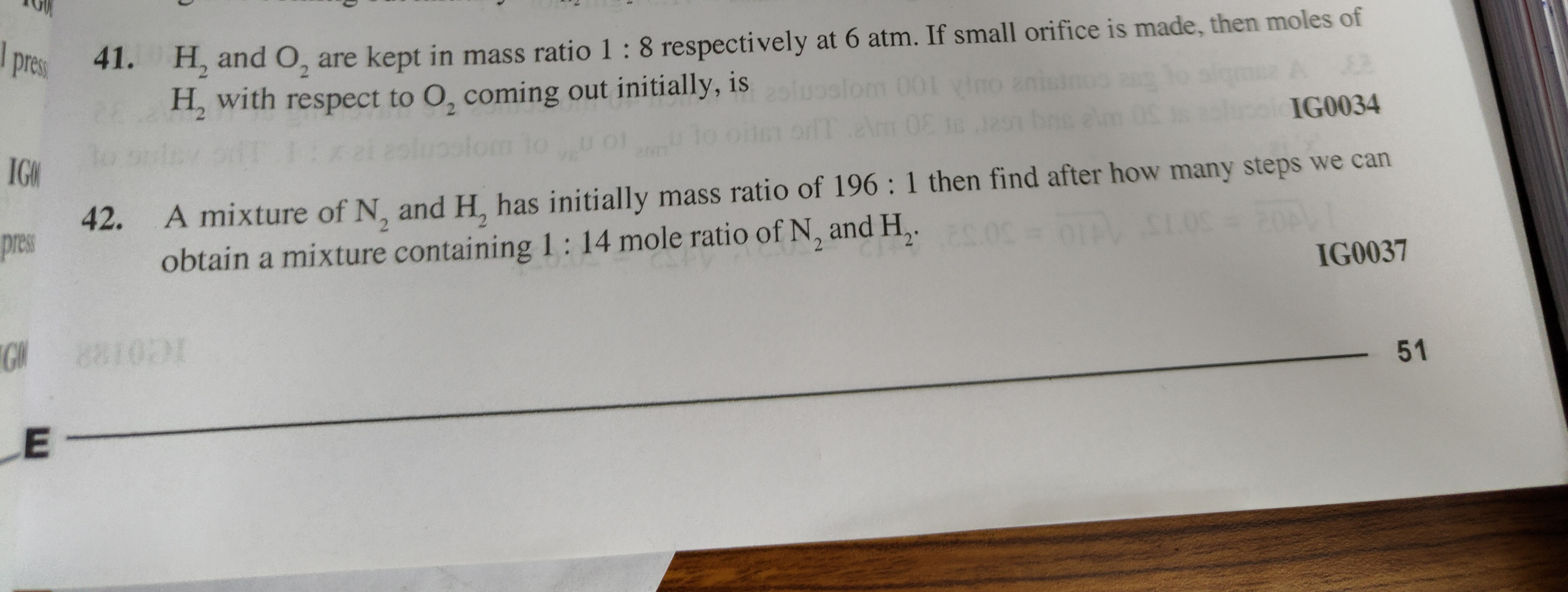
8:1
Solution
-
Calculate Molar Masses:
- Molar mass of H₂ (MH2) = 2 g/mol
- Molar mass of O₂ (MO2) = 32 g/mol
-
Determine Initial Mole Ratio: Let the mass of H₂ be mH2=x and the mass of O₂ be mO2=8x.
- Number of moles of H₂ (nH2) = mH2/MH2=x/2
- Number of moles of O₂ (nO2) = mO2/MO2=8x/32=x/4 The initial mole ratio is nH2:nO2=(x/2):(x/4)=2:1.
-
Calculate Partial Pressures: The total pressure (Ptotal) is 6 atm.
- Mole fraction of H₂ (XH2) = nH2/(nH2+nO2)=(x/2)/(x/2+x/4)=(x/2)/(3x/4)=2/3.
- Mole fraction of O₂ (XO2) = nO2/(nH2+nO2)=(x/4)/(3x/4)=1/3.
- Partial pressure of H₂ (PH2) = XH2×Ptotal=(2/3)×6 atm=4 atm.
- Partial pressure of O₂ (PO2) = XO2×Ptotal=(1/3)×6 atm=2 atm.
-
Apply Graham's Law of Effusion: The rate of effusion of a gas through a small orifice is proportional to its partial pressure and inversely proportional to the square root of its molar mass. Rate ∝MP The ratio of moles coming out initially is equal to the ratio of their rates of effusion. Moles of O₂ coming outMoles of H₂ coming out=Rate of O₂Rate of H₂=PO2PH2MH2MO2 Moles of O₂ coming outMoles of H₂ coming out=2 atm4 atm2 g/mol32 g/mol Moles of O₂ coming outMoles of H₂ coming out=216=2×4=8 The moles of H₂ with respect to O₂ coming out initially is 8:1.
