Question
Question: A substance is in the solid form at 0°C. The amount of heat added to this substance and it temperatu...
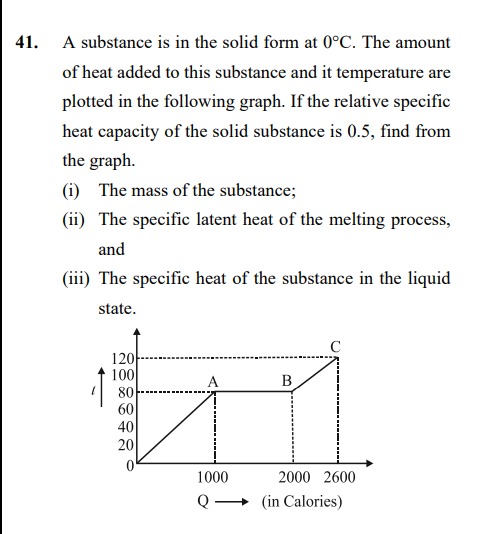
A substance is in the solid form at 0°C. The amount of heat added to this substance and it temperature are plotted in the following graph. If the relative specific heat capacity of the solid substance is 0.5, find from the graph. (i) The mass of the substance; (ii) The specific latent heat of the melting process, and (iii) The specific heat of the substance in the liquid state.
(i) The mass of the substance is 25 g. (ii) The specific latent heat of the melting process is 40 cal/g. (iii) The specific heat of the substance in the liquid state is 0.6 cal/g°C.
Solution
The graph shows three distinct processes:
- Region OA: Heating of the solid from 0°C to 80°C. Heat added is 1000 calories.
- Region AB: Melting of the substance at a constant temperature of 80°C. Heat added is 2000 - 1000 = 1000 calories.
- Region BC: Heating of the liquid from 80°C to 120°C. Heat added is 2600 - 2000 = 600 calories.
Assuming the relative specific heat capacity is with respect to water (cwater=1 cal/g°C), the specific heat of the solid is csolid=0.5 cal/g°C.
(i) Mass of the substance (m): In region OA, ΔQ=1000 cal and ΔT=80°C. Using Q=mcΔT: 1000 cal=m×(0.5 cal/g°C)×(80°C) 1000=40m m=401000=25 g.
(ii) Specific latent heat of melting (L): In region AB, the heat added for melting is ΔQ=1000 cal. Using Q=mL: 1000 cal=(25 g)×L L=251000=40 cal/g.
(iii) Specific heat of the substance in the liquid state (cliquid): In region BC, ΔQ=600 cal and ΔT=120°C−80°C=40°C. Using Q=mcΔT: 600 cal=(25 g)×cliquid×(40°C) 600=1000cliquid cliquid=1000600=0.6 cal/g°C.
