Question
Question: The decreasing order of reactivity of the following organic molecules towards $AgNO_3$ solution is...
The decreasing order of reactivity of the following organic molecules towards AgNO3 solution is
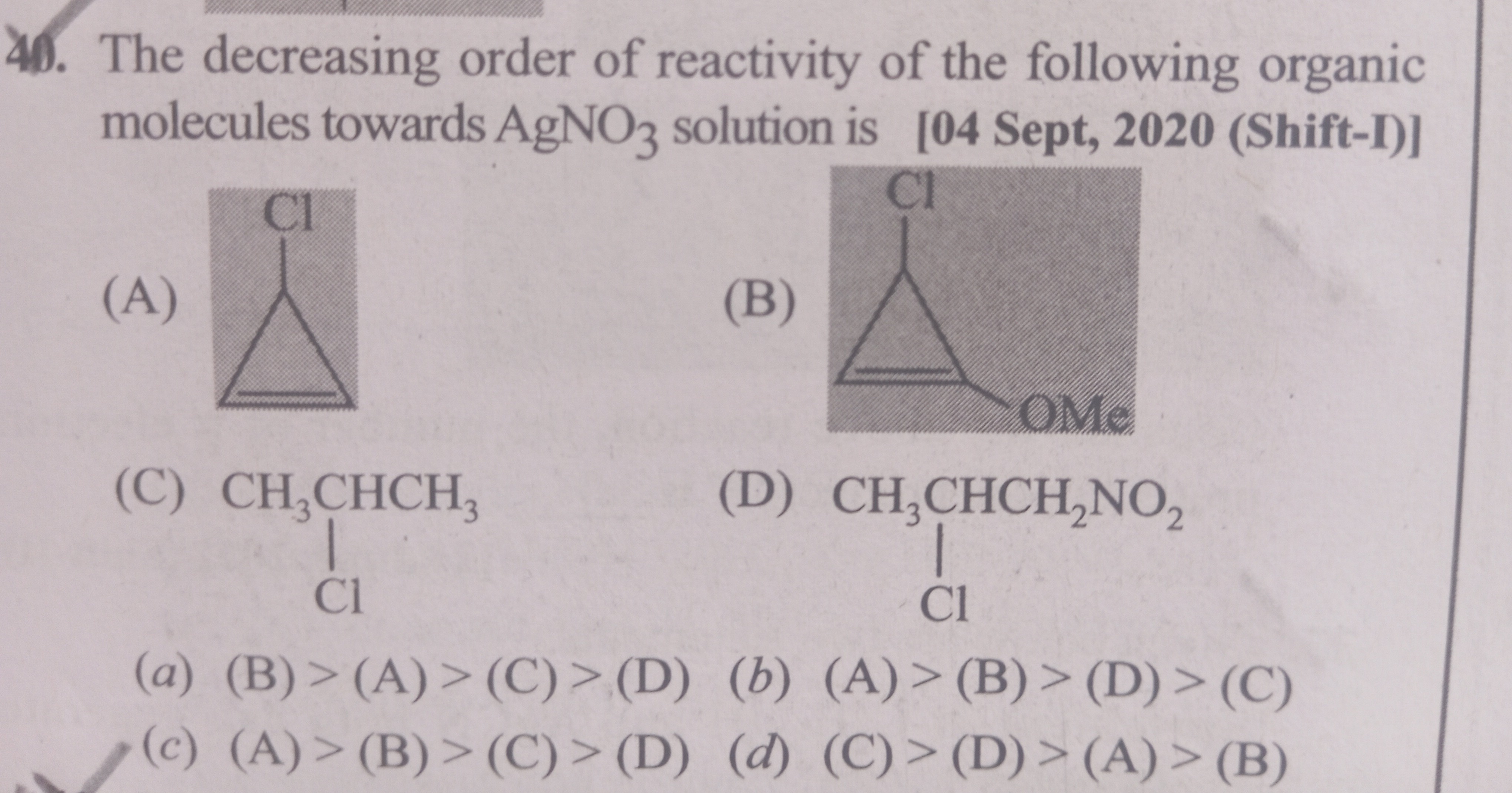
A
(B) > (A) > (C) > (D)
B
(A) > (B) > (D) > (C)
C
(A) > (B) > (C) > (D)
D
(C) > (D) > (A) > (B)
Answer
(B) > (A) > (C) > (D)
Explanation
Solution
Reactivity towards AgNO3 is an SN1 reaction, dependent on carbocation stability.
- (B): Forms a carbocation highly stabilized by the +M effect of the −OMe group. (Most stable)
- (A): Forms an allylic carbocation. Despite ring strain, allylic resonance provides significant stabilization, making it more stable than a simple secondary carbocation.
- (C): Forms a simple secondary carbocation, stabilized by hyperconjugation.
- (D): Forms a secondary carbocation destabilized by the strong -I effect of the β-NO2 group. (Least stable)
Therefore, the decreasing order of reactivity is (B) > (A) > (C) > (D).
