Question
Question: The compound $\qquad \qquad \qquad$O $\qquad \qquad \qquad ||$ HOCH$_{2}$CH$_{2}$CH$_{2}$CH$_{2}$CH...
The compound
O ∣∣ HOCH2CH2CH2CH2CH can form a hemiacetal by reacting with itself in solution. What is the structure of this hemiacetal?
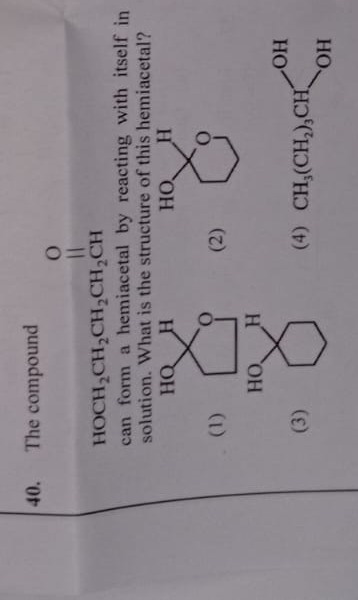
Option (1) shows a 5-membered ring. This would be formed if the hydroxyl group on C4 attacked the aldehyde. Since the hydroxyl group is on C5, this option is incorrect.
Option (2) shows a 6-membered ring containing one oxygen atom. The carbon adjacent to the oxygen within the ring (which corresponds to the original aldehyde carbon) has an -OH group and an -H atom attached. This perfectly matches the structure derived from the intramolecular reaction of 5-hydroxypentanal.
Option (3) shows a 6-membered carbon ring (cyclohexane derivative) with two hydroxyl groups. This is a cyclic diol, not a cyclic hemiacetal.
Option (4) shows a straight-chain gem-diol, CH3(CH2)3CH(OH)2, which is not a cyclic hemiacetal and also has a different carbon skeleton compared to the starting material after considering the aldehyde.
2
Solution
The given compound, 5-hydroxypentanal, has both an aldehyde group at C1 and a hydroxyl group at C5. An intramolecular reaction occurs where the hydroxyl oxygen at C5 attacks the electrophilic carbonyl carbon at C1. This forms a 6-membered ring consisting of 5 carbon atoms (C1-C5) and 1 oxygen atom (from the C5-OH group). The original carbonyl carbon (C1) becomes an anomeric carbon, bonded to an -OH group (from the original carbonyl oxygen) and an -H atom, consistent with a cyclic hemiacetal structure. Option (2) correctly depicts this 6-membered cyclic hemiacetal.
