Question
Question: Match the reaction intermediates formed during the reactions given in Column-I with Column-II | ...
Match the reaction intermediates formed during the reactions given in Column-I with Column-II
| Column-I | Column-II | |
|---|---|---|
| A | CH3−C≡C−HNa | P Carbocation (Non classical) |
| B | CH3−CH=CH2HBr | Q Carbocation (Classical) |
| C | CH3−CH=CH2HBrPeroxide | R Carbanion |
| D | CH3−CH=CH2Br2/CCl4S | S Alkyl free radical |
| A B C D |
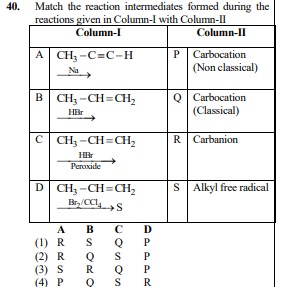
R S Q P
R Q S P
S R Q P
P Q S R
2
Solution
The problem asks us to match the given reactions with the reaction intermediates formed during them. We will analyze each reaction individually.
A. CH3−C≡C−HNa
This is the reaction of a terminal alkyne with sodium metal. Terminal alkynes have an acidic hydrogen. Sodium is a strong base and will deprotonate the alkyne, forming an acetylide anion.
CH3−C≡C−H+Na→CH3−C≡C−Na++21H2
The intermediate formed is CH3−C≡C−, which is a Carbanion.
Thus, A matches with R.
B. CH3−CH=CH2HBr
This is an electrophilic addition reaction of HBr to propene, following Markovnikov's rule. The first step involves the addition of a proton (H+) to the double bond, forming the more stable carbocation.
CH3−CH=CH2+H+→CH3−CH+−CH3 (secondary carbocation)
This is a carbon atom with a positive charge, which is a Classical Carbocation.
Thus, B matches with Q.
C. CH3−CH=CH2HBrPeroxide
This is the addition of HBr to propene in the presence of peroxide, which is an anti-Markovnikov addition (Kharasch effect) via a free radical mechanism. The propagation step involves the addition of a bromine radical (Br⋅) to the alkene, forming the more stable alkyl free radical.
CH3−CH=CH2+Br⋅→CH3−CH⋅−CH2Br (secondary alkyl radical)
The intermediate formed is an Alkyl free radical.
Thus, C matches with S.
D. CH3−CH=CH2Br2/CCl4
This is an electrophilic addition reaction of Br2 to propene. The mechanism involves the formation of a cyclic bromonium ion. The Br2 molecule is polarized, and one bromine atom attacks the double bond, forming a three-membered ring intermediate with a positive charge on the bromine atom. This bridged ion is considered a Non-classical Carbocation.
Thus, D matches with P.
Summary of Matches:
A - R
B - Q
C - S
D - P
Comparing this with the given options:
(1) R S Q P
(2) R Q S P
(3) S R Q P
(4) P Q S R
The correct sequence is R Q S P, which corresponds to option (2).
