Question
Question: Arrange the following alcohols in decreasing order of the ease of ionization under acidic conditions...
Arrange the following alcohols in decreasing order of the ease of ionization under acidic conditions.
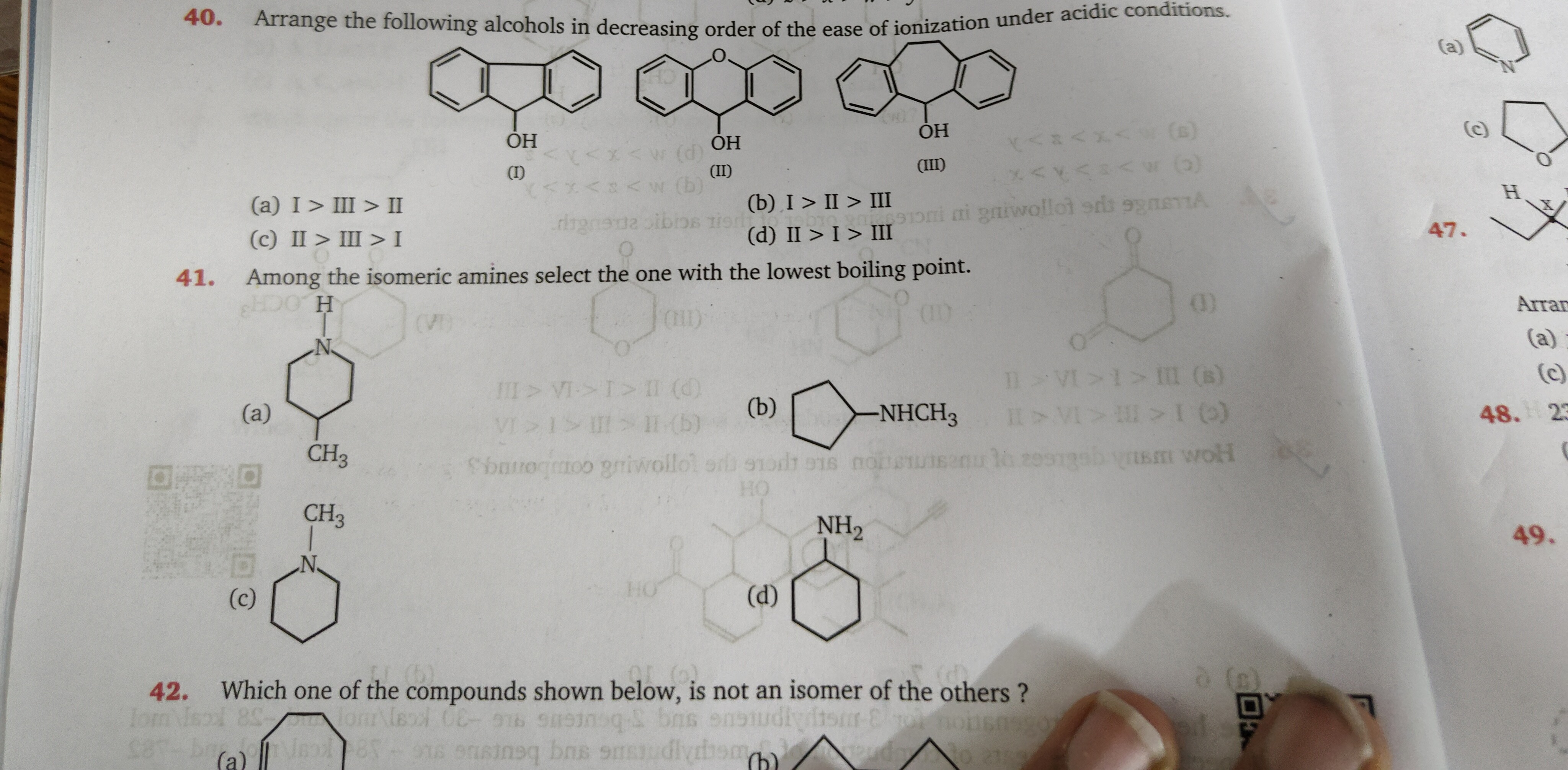
A
I > III > II
B
II > III > I
C
I > II > III
D
II > I > III
Answer
I > II > III
Explanation
Solution
The ease of ionization of an alcohol under acidic conditions depends on the stability of the carbocation formed after protonation of the hydroxyl group and loss of water. A more stable carbocation leads to easier ionization.
- Compound (I) (9H-fluoren-9-ol): Forms the fluorenyl carbocation, which is significantly resonance stabilized due to its aromatic nature.
- Compound (II) (9H-xanthen-9-ol): Forms the xanthenyl carbocation, stabilized by resonance into the phenyl rings and donation from the oxygen atom.
- Compound (III) (9H-anthracen-9-ol): Forms the anthracenyl carbocation, stabilized by resonance within the anthracene system.
The relative stability of these carbocations is: Fluorenyl > Xanthenyl > Anthracenyl. Therefore, the decreasing order of ease of ionization is I > II > III.
