Question
Question: 6 gm nitrogen on successive reaction with different compounds gets finally converted into [Cr(NH$_3$...
6 gm nitrogen on successive reaction with different compounds gets finally converted into [Cr(NH3)xBr2]. Value of x is [Atomic mass : Cr = 52, Br = 80]
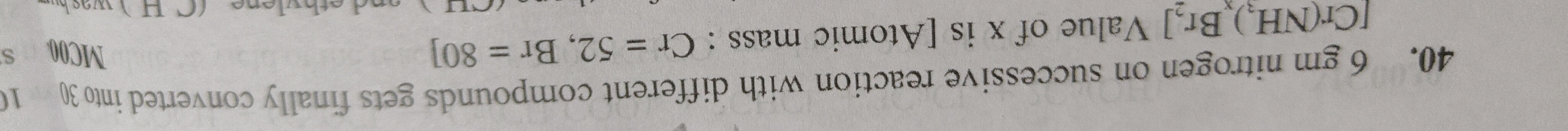
Answer
4
Explanation
Solution
The complex is [Cr(NH3)xBr2]. Chromium typically forms coordination compounds with a coordination number of 6. NH3 and Br are monodentate ligands. Thus, the sum of the number of NH3 ligands (x) and Br ligands (2) must equal the coordination number 6. So, x + 2 = 6, which gives x = 4. The 6 gm nitrogen information is irrelevant for determining x.
