Question
Question: Which of the following Ist ionisation energy order is/are correct...
Which of the following Ist ionisation energy order is/are correct
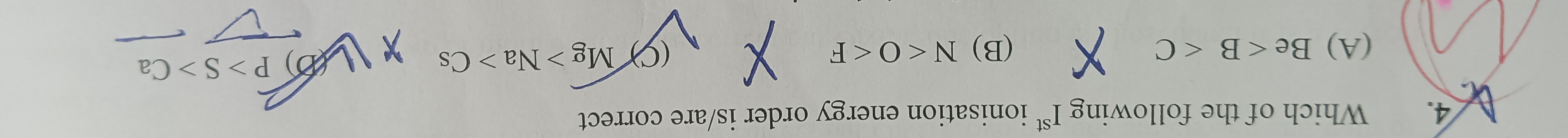
A
Be < B < C X
B
N < O < F X
C
Mg > Na > Cs X
D
P > S > Ca
Answer
C, D
Explanation
Solution
The first ionization energy (IE) is the energy required to remove the most loosely held electron from a gaseous atom. The trends in ionization energy are as follows:
- Across a period (left to right): Generally increases due to increasing nuclear charge and decreasing atomic size.
- Down a group (top to bottom): Generally decreases due to increasing atomic size and shielding effect.
Exceptions to the general trend across a period:
- Group 2 (ns²) vs Group 13 (ns²np¹): Elements in Group 2 have fully filled s-orbitals, which are more stable. Thus, IE(Group 2) > IE(Group 13) in the same period (e.g., Be > B, Mg > Al).
- Group 15 (ns²np³) vs Group 16 (ns²np⁴): Elements in Group 15 have half-filled p-orbitals, which are more stable. Thus, IE(Group 15) > IE(Group 16) in the same period (e.g., N > O, P > S).
Let's analyze each option:
(A) Be < B < C
- Be (Group 2, Period 2): Electronic configuration 1s²2s². Stable fully-filled 2s orbital.
- B (Group 13, Period 2): Electronic configuration 1s²2s²2p¹.
- C (Group 14, Period 2): Electronic configuration 1s²2s²2p².
- Due to the stability of the fully-filled 2s orbital, Be has a higher IE than B. So, IE(Be) > IE(B).
- The correct order for these three is B < Be < C.
- Therefore, option (A) is incorrect.
(B) N < O < F
- N (Group 15, Period 2): Electronic configuration 1s²2s²2p³. Stable half-filled 2p orbital.
- O (Group 16, Period 2): Electronic configuration 1s²2s²2p⁴.
- F (Group 17, Period 2): Electronic configuration 1s²2s²2p⁵.
- Due to the stability of the half-filled 2p orbital, N has a higher IE than O. So, IE(N) > IE(O).
- The correct order for these three is O < N < F.
- Therefore, option (B) is incorrect.
(C) Mg > Na > Cs
- Mg vs Na: Both are in Period 3. Na is in Group 1, Mg is in Group 2. Due to the stable fully-filled 3s orbital of Mg and higher nuclear charge, IE(Mg) > IE(Na). (Correct)
- Na vs Cs: Both are in Group 1. Na is in Period 3, Cs is in Period 6. As we move down a group, atomic size increases and shielding effect increases, leading to a decrease in IE. So, IE(Na) > IE(Cs). (Correct)
- Combining these, the order is IE(Mg) > IE(Na) > IE(Cs).
- Therefore, option (C) is correct.
(D) P > S > Ca
- P vs S: Both are in Period 3. P is in Group 15 (3s²3p³), S is in Group 16 (3s²3p⁴). Due to the stable half-filled 3p orbital, IE(P) > IE(S). (Correct)
- S vs Ca: S is in Period 3, Group 16. Ca is in Period 4, Group 2.
- IE(S) = 999.6 kJ/mol
- IE(Ca) = 589.8 kJ/mol
- Since S is a non-metal and further to the right in its period, and Ca is an alkaline earth metal in a later period, IE(S) is significantly higher than IE(Ca). So, IE(S) > IE(Ca). (Correct)
- Combining these, the order is IE(P) > IE(S) > IE(Ca).
- Therefore, option (D) is correct.
Both options (C) and (D) are correct.
