Question
Question: When two equal sized pieces of the same metal at different temperatures $T_h$(hot piece) and $T_c$ (...
When two equal sized pieces of the same metal at different temperatures Th(hot piece) and Tc (cold piece) brought into contact into thermal contact and isolated from it's surrounding. The total change in entropy system is given by ? [Cv (J/K) = heat capacity of metal]
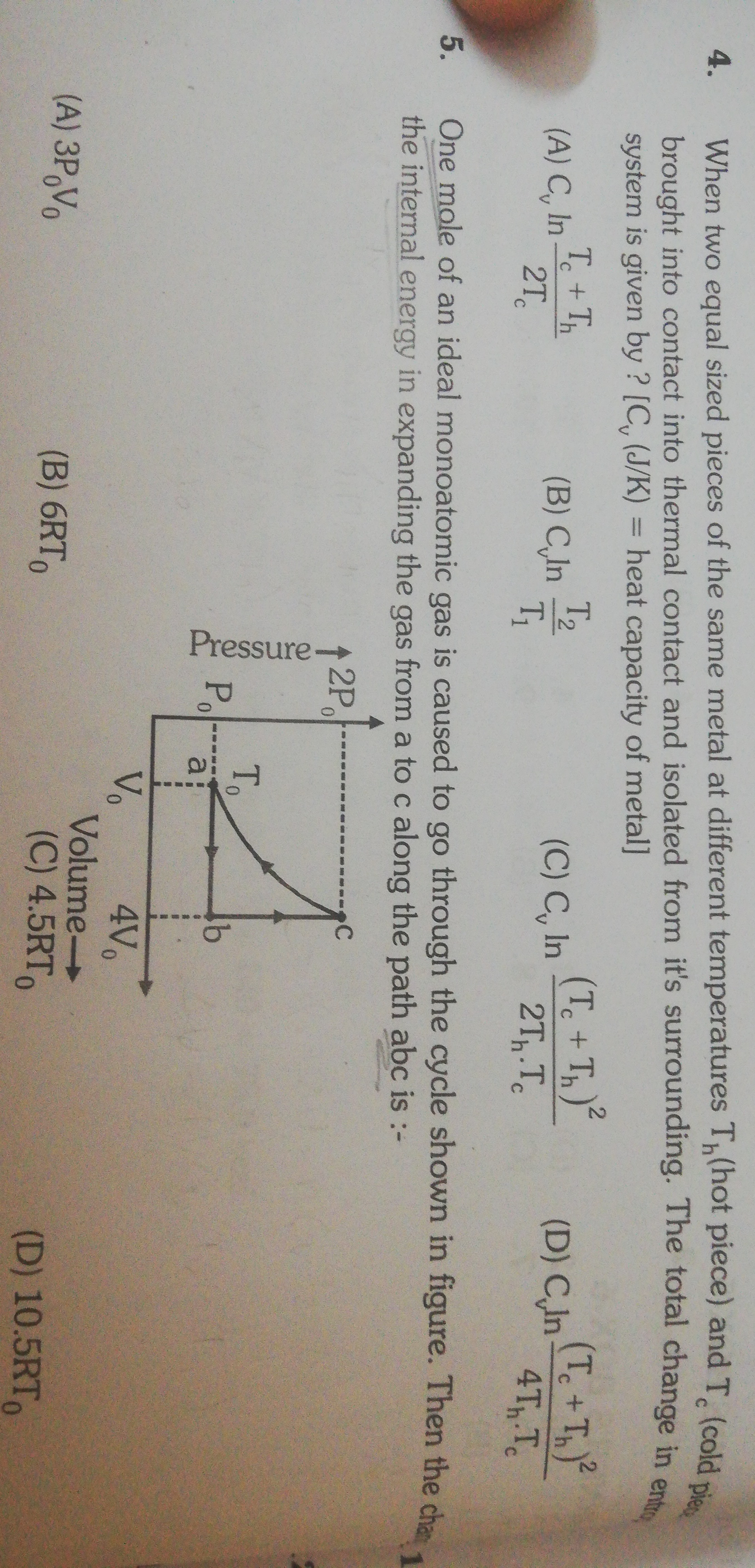
Cvln2TcTc+Th
CvlnT1T2
Cvln2Th⋅Tc(Tc+Th)2
Cvln4Th⋅Tc(Tc+Th)2
Cvln4Th⋅Tc(Tc+Th)2
Solution
The final equilibrium temperature Tf is found by equating heat lost by the hot piece to heat gained by the cold piece: Cv(Th−Tf)=Cv(Tf−Tc), yielding Tf=2Th+Tc. The entropy change for each piece is ΔS=Cvln(Tfinal/Tinitial). Thus, ΔShot=Cvln(Tf/Th) and ΔScold=Cvln(Tf/Tc). The total entropy change is the sum: ΔStotal=Cvln(Tf/Th)+Cvln(Tf/Tc)=Cvln(ThTcTf2). Substituting Tf gives the final result.
