Question
Question: What is value of PV type of work for following reaction at 1 bar? $C_2H_{4(g)}$ + $HCl_{(g)}$ → $C_...
What is value of PV type of work for following reaction at 1 bar?
C2H4(g) + HCl(g) → C2H5Cl(g) V1=0.3 0.2(200 mL) 0.15(150 mL) V2=0.15 ∴ first prreactant
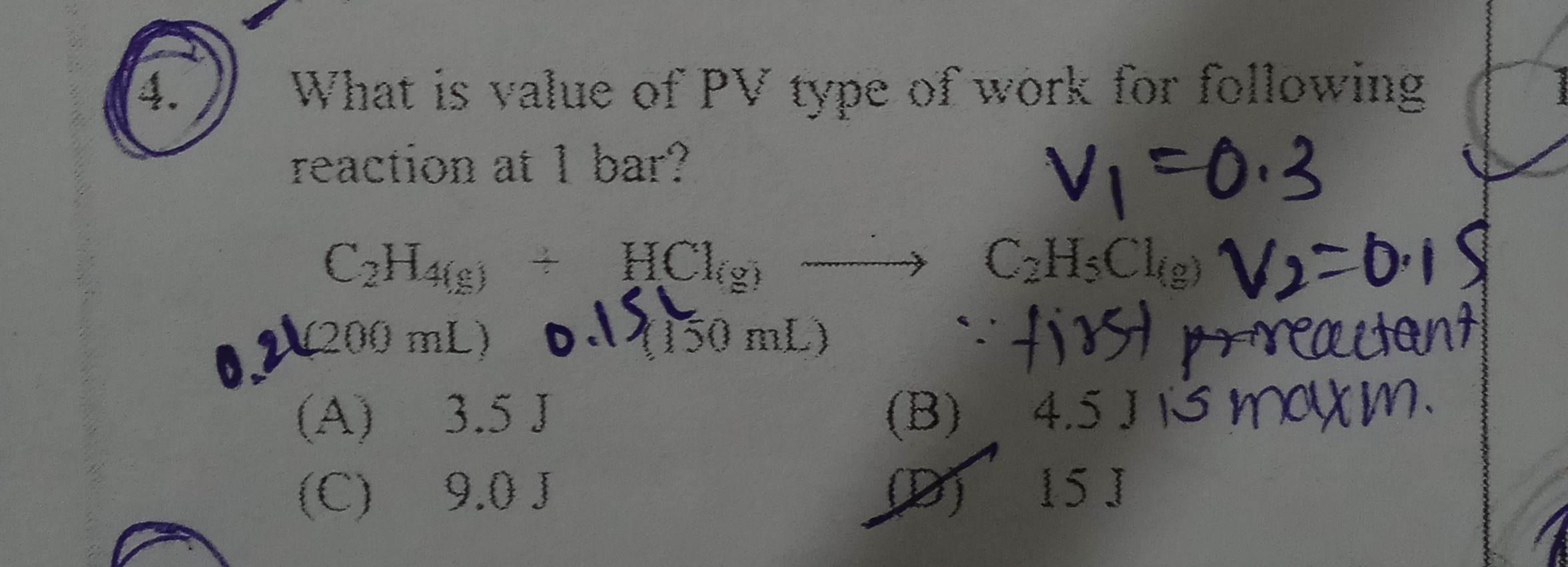
3.5 J
4.5 J
9.0 J
15 J
15 J
Solution
For the reaction
\ceC2H4(g)+HCl(g)−>C2H5Cl(g)the data given are:
- Ethylene: 200 mL (i.e. “0.2”)
- HCl: 150 mL (i.e. “0.15”)
Since the reaction uses 1 mole of each gas to form 1 mole of product gas, HCl (150 mL) is the limiting reactant. Thus, the reaction consumes 150 mL of ethylene (from the available 200 mL) so that 50 mL of ethylene remains unreacted.
Initial total gas volume
= 200 mL (ethylene) + 150 mL (HCl)
= 350 mL
Final total gas volume
= 150 mL (C₂H₅Cl formed) + 50 mL (unreacted ethylene)
= 200 mL
Therefore, the change in volume is
ΔV=Vfinal−Vinitial=200mL−350mL=−150mL=−0.15LAt constant pressure (1 bar), the work done in a PV work process is given by
w=−PΔVSubstituting,
w=−(1bar)×(−0.15L)=0.15LbarUsing the conversion factor 1Lbar≈100J,
w≈0.15×100J=15J