Question
Question: The correct order of boiling point is ...
The correct order of boiling point is
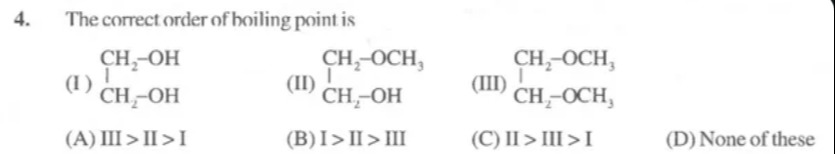
III > II > I
I > II > III
II > III > I
None of these
I > II > III
Solution
The boiling point of a substance is determined by the strength of the intermolecular forces between its molecules. Stronger intermolecular forces require more energy to overcome, resulting in a higher boiling point. The main intermolecular forces to consider here are hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
Let's examine the structures of the given compounds:
(I) Ethane-1,2-diol (Ethylene glycol): HO-CH2-CH2-OH
This molecule has two hydroxyl (-OH) groups. Hydroxyl groups are capable of forming strong intermolecular hydrogen bonds. Due to the presence of two -OH groups, ethane-1,2-diol can form extensive hydrogen bonding networks.
(II) 2-Methoxyethanol: CH3-O-CH2-CH2-OH
This molecule has one hydroxyl (-OH) group and one ether (-O-) group. The hydroxyl group can form intermolecular hydrogen bonds. The ether group is polar due to the electronegativity difference between oxygen and carbon, contributing to dipole-dipole interactions.
(III) 1,2-Dimethoxyethane: CH3-O-CH2-CH2-O-CH3
This molecule has two ether (-O-) groups. There are no hydrogen atoms directly bonded to oxygen, so it cannot form intermolecular hydrogen bonds among its own molecules. The main intermolecular forces are dipole-dipole interactions due to the polar C-O bonds and London dispersion forces.
Comparing the strengths of intermolecular forces:
Hydrogen bonding is the strongest type of intermolecular force among these. Dipole-dipole interactions are weaker than hydrogen bonding. London dispersion forces are present in all molecules and increase with molecular size and surface area.
Compound (I) has two -OH groups, allowing for extensive intermolecular hydrogen bonding.
Compound (II) has one -OH group, allowing for less extensive intermolecular hydrogen bonding compared to (I). It also has dipole-dipole interactions.
Compound (III) has no -OH groups, so no intermolecular hydrogen bonding. It has dipole-dipole interactions and London dispersion forces.
The strength of intermolecular forces is expected to be in the order: (I) > (II) > (III), primarily due to the difference in the extent of hydrogen bonding. This order of intermolecular forces directly translates to the order of boiling points.
Therefore, the correct order of boiling points is (I) > (II) > (III).
Let's confirm with actual boiling points:
Ethylene glycol (I): 197.3 °C
2-Methoxyethanol (II): 124.5 °C
1,2-Dimethoxyethane (III): 85 °C
The actual boiling points confirm the order I > II > III.
