Question
Question: Match the column...
Match the column
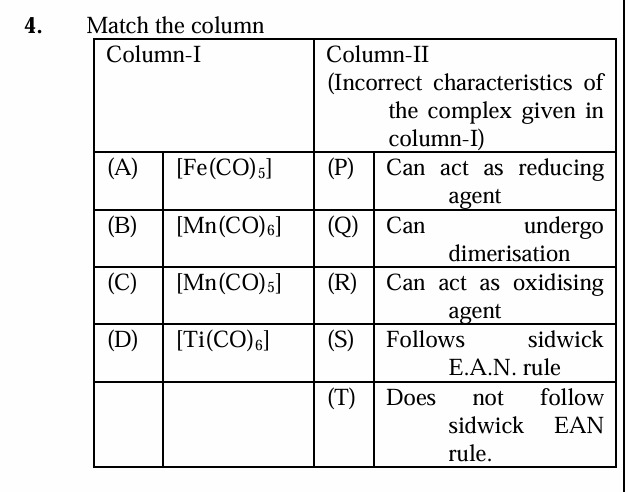
[Fe(CO)5] - (T) Does not follow sidwick EAN rule
[Mn(CO)6] - (S) Follows sidwick EAN rule
[Mn(CO)5] - (R) Can act as oxidising agent
[Ti(CO)6] - (Q) Can undergo dimerisation
[Fe(CO)5] - (Q) Can undergo dimerisation
[Fe(CO)5] - (R) Can act as oxidising agent
[Mn(CO)6] - (P) Can act as reducing agent
[Mn(CO)6] - (Q) Can undergo dimerisation
[Mn(CO)5] - (S) Follows sidwick EAN rule
[Mn(CO)5] - (P) Can act as reducing agent
[Ti(CO)6] - (S) Follows sidwick EAN rule
[Ti(CO)6] - (R) Can act as oxidising agent
[Ti(CO)6] - (P) Can act as reducing agent
(A-T), (B-S), (C-R), (D-Q)
Solution
The question asks to match each complex in Column-I with an incorrect characteristic from Column-II.
Analysis of Complexes:
-
[Fe(CO)5]:
- Iron (Fe) has atomic number Z=26.
- In neutral metal carbonyls, the oxidation state of the metal is 0.
- EAN = Z−Oxidation State+2×(Number of ligands)
- EAN = 26−0+2×5=26+10=36.
- Since 36 is the atomic number of Krypton (a noble gas), [Fe(CO)5] follows the Sidgwick EAN rule.
- Therefore, the statement (T) "Does not follow sidwick EAN rule" is INCORRECT for [Fe(CO)5].
- [Fe(CO)5] is a stable 18-electron complex and is not prone to dimerisation, so (Q) is correct. It can act as a reducing agent, so (P) is correct. It is unlikely to act as an oxidizing agent, so (R) is incorrect. However, (T) is the most direct incorrect characteristic related to the EAN rule.
-
[Mn(CO)6]:
- Manganese (Mn) has atomic number Z=25.
- Oxidation state of Mn is 0.
- EAN = 25−0+2×6=25+12=37.
- Since 37 is not the atomic number of a noble gas, [Mn(CO)6] does NOT follow the Sidgwick EAN rule.
- Therefore, the statement (S) "Follows sidwick EAN rule" is INCORRECT for [Mn(CO)6].
- [Mn(CO)6] is a 24-electron species and is not expected to dimerise readily, so (Q) is correct. It can act as a reducing agent, so (P) is correct. It is unlikely to act as an oxidizing agent, so (R) is incorrect. However, (S) is the most direct incorrect characteristic related to the EAN rule.
-
[Mn(CO)5]:
- Manganese (Mn) has atomic number Z=25.
- Oxidation state of Mn is 0.
- EAN = 25−0+2×5=25+10=35.
- Since 35 is not the atomic number of a noble gas, [Mn(CO)5] does NOT follow the Sidgwick EAN rule.
- This complex is a 17-electron radical and readily dimerises to form [Mn2(CO)10]. So, (Q) "Can undergo dimerisation" is correct.
- Metals in low oxidation states (like 0) typically act as reducing agents and are unlikely to act as oxidizing agents.
- Therefore, the statement (R) "Can act as oxidising agent" is INCORRECT for [Mn(CO)5].
- The statement (S) "Follows sidwick EAN rule" is also incorrect. However, (R) is a distinct property.
-
[Ti(CO)6]:
- Titanium (Ti) has atomic number Z=22.
- Oxidation state of Ti is 0.
- EAN = 22−0+2×6=22+12=34.
- Since 34 is not the atomic number of a noble gas, [Ti(CO)6] does NOT follow the Sidgwick EAN rule.
- This complex is a 24-electron species and is generally considered not to readily dimerise.
- Therefore, the statement (Q) "Can undergo dimerisation" is INCORRECT for [Ti(CO)6].
- The statement (S) "Follows sidwick EAN rule" is also incorrect. It is unlikely to act as an oxidizing agent, so (R) is incorrect. However, (Q) is a distinct property that is incorrect.
Conclusion: The matches for incorrect characteristics are:
- [Fe(CO)5] - (T)
- [Mn(CO)6] - (S)
- [Mn(CO)5] - (R)
- [Ti(CO)6] - (Q)
This set provides a unique match for each item from Column-I with an option from Column-II, using each option from Column-II exactly once.
