Question
Question: \[4\]-Hydroxy- \[4\]-methyl pentanal on heating with excess of methanol in the presence of an acid c...
4-Hydroxy- 4-methyl pentanal on heating with excess of methanol in the presence of an acid catalyst followed by dehydration of the product gives
A)
B)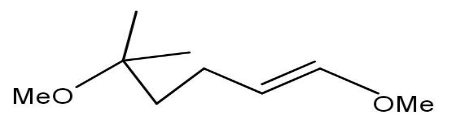
C)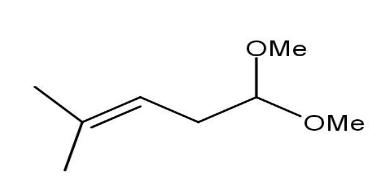
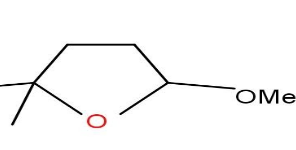
D)
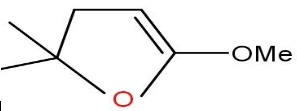
Solution
The carbonyl compounds when heated with excess methanol then two methoxy groups were inserted on the carbonyl carbon.
When alcohols also known as hydroxy compounds undergo dehydration, they form alkenes.
Complete answer: Given compound is 4−hydroxy −4−methylpentanal.
The parent chain is pentanal, it has five carbon atoms. There is a substitution at fourth carbon.
The numbering should be started from aldehydic carbon.
There are both methyl and hydroxyl groups on 4th carbon.
When given compound treats with excess methanol, then the aldehydic carbon converts into the carbon containing methoxy and hydroxyl groups.
As it is treated with excess methanol, the hydroxyl group also converts into methoxy group.
(CH3)2C(OH)CH2CH2CH(OH)(CH3)→(CH3)2C(OH)CH2CH2CH(OCH3)2
Now the above product, treated with an acid catalyst, undergoes dehydration.
Dehydration is defined as the loss of water molecules.
The hydroxyl group on 4th carbon and hydrogen on 3rd carbon ate lost as a water molecule.
Thus, the 4th carbon gets positive charge, and the third carbon gets negative charge.
Thereby forms an alkene.
Alkene is an unsaturated organic compound with the presence of a double bond.
Thus, when the given compound undergoes heating with excess methanol in the presence of acid catalyst followed by dehydration it forms a compound namely1,1−dimethoxy−4−methyl 3−pentene.
Option A will not be formed as the methylation cannot be done on 1st carbon.
Option C and D are also not formed as the compound does not undergo cyclisation.
Thus, option B is the correct one.
Note:
The methylation should be done on the aldehydic carbon only. At first one methoxy group attacks on aldehydic carbon, and then due to excess methanol the hydroxyl group can also convert to methoxy group.
The water molecule can be lost from the neighboring carbon atoms only.
