Question
Question: How many compounds are polar, planar and underlined atom is $sp^3d$ hybridised $\underline{PCl_3F_2...
How many compounds are polar, planar and underlined atom is sp3d hybridised
PCl3F2, PF3Cl2, XeF2, ClF3, BrF5, I3−, SF4, S3O9
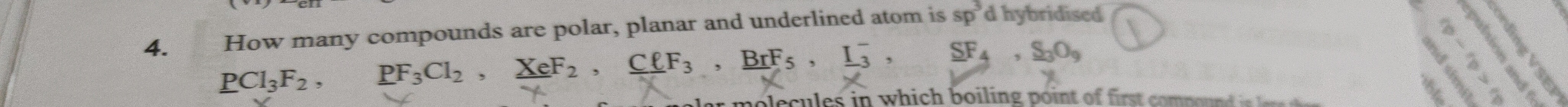
Answer
1
Explanation
Solution
To determine which compounds are polar, planar, and have the underlined atom sp3d hybridised, we will analyze each compound based on its Lewis structure, VSEPR theory, and molecular geometry.
Requirements:
- Hybridization: The central (underlined) atom must be sp3d hybridized. This means its steric number (number of bonded atoms + number of lone pairs) must be 5.
- Planarity: All atoms in the molecule must lie in the same plane.
- Polarity: The molecule must have a net dipole moment (i.e., it must be asymmetric in terms of bond dipoles and lone pair contributions).
Let's examine each compound:
-
PCl3F2
- Underlined atom: P
- Valence electrons of P: 5
- Bonded atoms: 5 (3 Cl, 2 F)
- Lone pairs: 0
- Steric Number (SN): 5 + 0 = 5.
- Hybridization: sp3d (Matches requirement).
- Electron Geometry: Trigonal bipyramidal (TBP).
- Molecular Geometry: Trigonal bipyramidal. In TBP, more electronegative atoms (F) prefer axial positions, and less electronegative atoms (Cl) prefer equatorial positions. This results in a 3D structure.
- Planarity: Not planar. The axial F atoms are out of the plane of the equatorial Cl atoms.
- Polarity: Due to the presence of different types of atoms (Cl and F) and their asymmetric arrangement (F axial, Cl equatorial), the bond dipoles do not cancel out. The molecule is polar.
- Conclusion: Fails planarity.
-
PF3Cl2
- Underlined atom: P
- Valence electrons of P: 5
- Bonded atoms: 5 (3 F, 2 Cl)
- Lone pairs: 0
- Steric Number (SN): 5 + 0 = 5.
- Hybridization: sp3d (Matches requirement).
- Electron Geometry: Trigonal bipyramidal (TBP).
- Molecular Geometry: Trigonal bipyramidal. The two less electronegative Cl atoms occupy equatorial positions. The three F atoms occupy one equatorial and two axial positions. This is a 3D structure.
- Planarity: Not planar. The axial F atoms are out of the plane of the equatorial atoms (Cl, Cl, F).
- Polarity: Due to different atoms (Cl and F) and an asymmetric arrangement, the bond dipoles do not cancel. The molecule is polar.
- Conclusion: Fails planarity.
-
XeF2
- Underlined atom: Xe
- Valence electrons of Xe: 8
- Bonded atoms: 2 (2 F)
- Lone pairs: (8 - 2*1) / 2 = 3 lone pairs.
- Steric Number (SN): 2 + 3 = 5.
- Hybridization: sp3d (Matches requirement).
- Electron Geometry: Trigonal bipyramidal (TBP). Lone pairs occupy equatorial positions to minimize repulsions.
- Molecular Geometry: Linear (F-Xe-F). The three lone pairs are in the equatorial plane, and the two F atoms are axial.
- Planarity: A linear molecule is always planar. (Matches requirement).
- Polarity: The molecule is perfectly symmetrical (F-Xe-F). The bond dipoles cancel out. The molecule is nonpolar.
- Conclusion: Fails polarity.
-
ClF3
- Underlined atom: Cl
- Valence electrons of Cl: 7
- Bonded atoms: 3 (3 F)
- Lone pairs: (7 - 3*1) / 2 = 2 lone pairs.
- Steric Number (SN): 3 + 2 = 5.
- Hybridization: sp3d (Matches requirement).
- Electron Geometry: Trigonal bipyramidal (TBP). Lone pairs occupy equatorial positions.
- Molecular Geometry: T-shaped. The two lone pairs are in the equatorial plane, and two F atoms are axial, one F atom is equatorial.
- Planarity: All four atoms (Cl and 3 F) lie in the same plane. (Matches requirement).
- Polarity: The T-shaped geometry is asymmetric. The bond dipoles do not cancel, and the lone pairs contribute to the net dipole moment. The molecule is polar. (Matches requirement).
- Conclusion: Satisfies all conditions.
-
BrF5
- Underlined atom: Br
- Valence electrons of Br: 7
- Bonded atoms: 5 (5 F)
- Lone pairs: (7 - 5*1) / 2 = 1 lone pair.
- Steric Number (SN): 5 + 1 = 6.
- Hybridization: sp3d2 (Does not match sp3d).
- Conclusion: Fails hybridization.
-
I3−
- Underlined atom: Central I
- Valence electrons of central I: 7 (plus 1 for the negative charge) = 8 electrons.
- Bonded atoms: 2 (2 terminal I atoms)
- Lone pairs: (8 - 2*1) / 2 = 3 lone pairs.
- Steric Number (SN): 2 + 3 = 5.
- Hybridization: sp3d (Matches requirement).
- Electron Geometry: Trigonal bipyramidal (TBP). Lone pairs occupy equatorial positions.
- Molecular Geometry: Linear (I-I-I). The three lone pairs are in the equatorial plane, and the two terminal I atoms are axial.
- Planarity: A linear molecule is always planar. (Matches requirement).
- Polarity: The molecule is perfectly symmetrical (I-I-I). The bond dipoles cancel out. The molecule is nonpolar.
- Conclusion: Fails polarity.
-
SF4
- Underlined atom: S
- Valence electrons of S: 6
- Bonded atoms: 4 (4 F)
- Lone pairs: (6 - 4*1) / 2 = 1 lone pair.
- Steric Number (SN): 4 + 1 = 5.
- Hybridization: sp3d (Matches requirement).
- Electron Geometry: Trigonal bipyramidal (TBP). The lone pair occupies an equatorial position.
- Molecular Geometry: See-saw. Two F atoms are axial, and two F atoms are equatorial.
- Planarity: Not planar. The axial F atoms are out of the plane of the equatorial F atoms and the central S atom.
- Polarity: The see-saw geometry is asymmetric due to the lone pair and the different positions of the F atoms. The bond dipoles do not cancel. The molecule is polar.
- Conclusion: Fails planarity.
-
S3O9
- No atom is underlined, so this compound is not to be evaluated for the given criteria.
Only ClF3 satisfies all three conditions.
Explanation of the solution:
- PCl3F2: Steric number 5 (sp3d). Trigonal bipyramidal geometry. Not planar. Polar. (Fails planarity)
- PF3Cl2: Steric number 5 (sp3d). Trigonal bipyramidal geometry. Not planar. Polar. (Fails planarity)
- XeF2: Steric number 5 (sp3d). Linear geometry. Planar. Nonpolar (symmetrical). (Fails polarity)
- ClF3: Steric number 5 (sp3d). T-shaped geometry. Planar. Polar (asymmetric). (Satisfies all)
- BrF5: Steric number 6 (sp3d2). (Fails hybridization)
- I3-: Steric number 5 (sp3d). Linear geometry. Planar. Nonpolar (symmetrical). (Fails polarity)
- SF4: Steric number 5 (sp3d). See-saw geometry. Not planar. Polar. (Fails planarity)
Only ClF3 meets all criteria.
