Question
Question: For a real gas (mol. mass = 30) if density at critical point is 0.40 g/cm³ and its T$\_c$ = $\frac{1...
For a real gas (mol. mass = 30) if density at critical point is 0.40 g/cm³ and its T_c = 41104K, then calculate Van der Waals constant a (in atm L2mol−2).
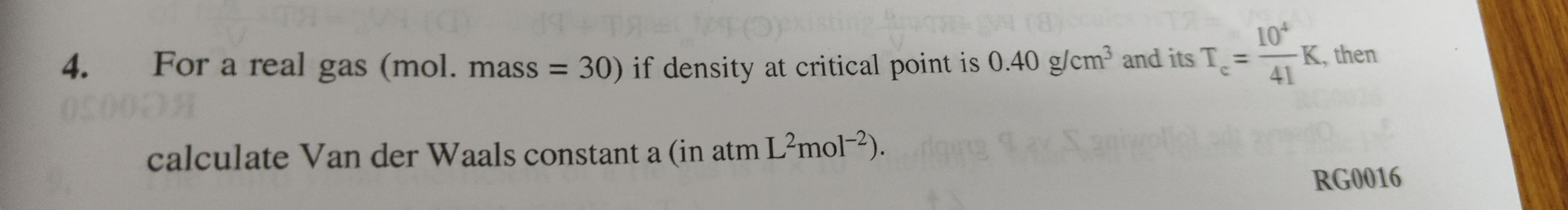
Answer
0.633 atm L²mol⁻²
Explanation
Solution
The relationship between the Van der Waals constant 'a' and the critical parameters is given by: a=64ρc27RTcM Where:
- R is the universal gas constant (0.0821 L atm K⁻¹ mol⁻¹)
- Tc is the critical temperature (41104 K)
- M is the molar mass (30 g/mol)
- ρc is the critical density (0.40 g/cm³ = 400 g/L)
Substituting the values: a=64×(400 g/L)27×(0.0821 L atm K⁻¹ mol⁻¹)×(41104 K)×(30 g/mol) a=41×64×40027×0.0821×10000×30 a≈0.633 atm L2mol−2
