Question
Question: 50 gm of $CaCO_3$ is allowed to react with 68.6 gm of $H_3PO_4$ then select the correct option(s)- $...
50 gm of CaCO3 is allowed to react with 68.6 gm of H3PO4 then select the correct option(s)- 3CaCO3+2H3PO4⟶Ca3(PO4)2+3H2O+3CO2
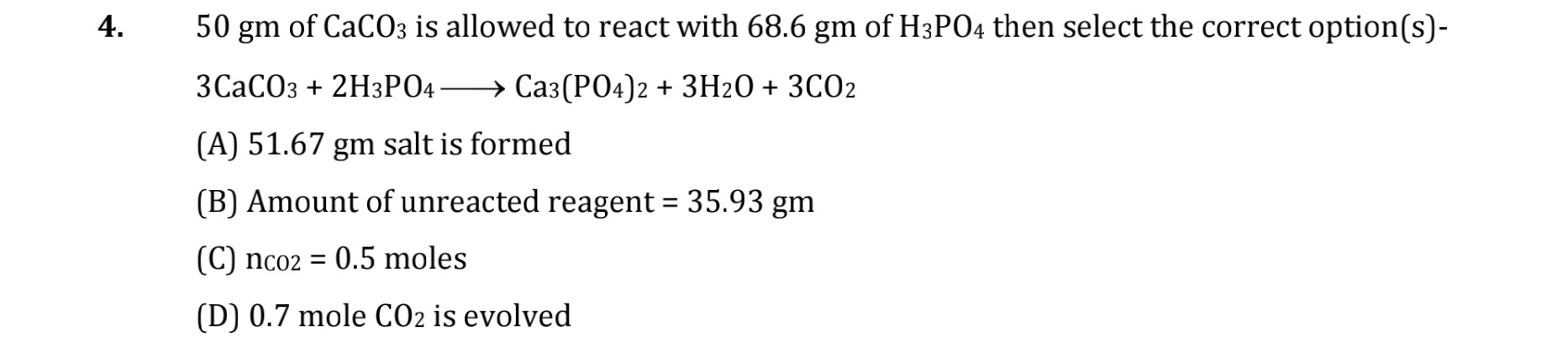
51.67 gm salt is formed
Amount of unreacted reagent = 35.93 gm
nCO2 = 0.5 moles
0.7 mole CO2 is evolved
A, B, C
Solution
The problem involves a stoichiometry calculation for the reaction between calcium carbonate (CaCO3) and phosphoric acid (H3PO4).
1. Balanced Chemical Equation: The given balanced chemical equation is: 3CaCO3+2H3PO4⟶Ca3(PO4)2+3H2O+3CO2
2. Molar Masses of Reactants and Products:
- Molar mass of CaCO3=40+12+(3×16)=100 g/mol
- Molar mass of H3PO4=(3×1)+31+(4×16)=3+31+64=98 g/mol
- Molar mass of Ca3(PO4)2=(3×40)+2×(31+(4×16))=120+2×(31+64)=120+2×95=120+190=310 g/mol
- Molar mass of CO2=12+(2×16)=12+32=44 g/mol
3. Calculate Initial Moles of Reactants:
- Moles of CaCO3=Molar massGiven mass=100 gm/mol50 gm=0.5 mol
- Moles of H3PO4=Molar massGiven mass=98 gm/mol68.6 gm=0.7 mol
4. Identify the Limiting Reagent: To find the limiting reagent, divide the moles of each reactant by its stoichiometric coefficient in the balanced equation:
- For CaCO3: 30.5 mol=0.1667
- For H3PO4: 20.7 mol=0.35
Since 0.1667<0.35, CaCO3 is the limiting reagent. It will be completely consumed, and all calculations for products and unreacted reagent will be based on the amount of CaCO3.
5. Evaluate Each Option:
(A) 51.67 gm salt (Ca3(PO4)2) is formed: From the balanced equation, 3 moles of CaCO3 produce 1 mole of Ca3(PO4)2. Moles of Ca3(PO4)2 formed = 0.5 mol CaCO3×3 mol CaCO31 mol Ca3(PO4)2=30.5=0.1666... mol Mass of Ca3(PO4)2 formed = 0.1666... mol×310 gm/mol=51.666... gm≈51.67 gm So, option (A) is correct.
(B) Amount of unreacted reagent = 35.93 gm: The unreacted reagent is H3PO4. From the balanced equation, 3 moles of CaCO3 react with 2 moles of H3PO4. Moles of H3PO4 reacted = 0.5 mol CaCO3×3 mol CaCO32 mol H3PO4=31=0.3333... mol Moles of H3PO4 unreacted = Initial moles - Moles reacted =0.7 mol−0.3333... mol=0.3666... mol Mass of unreacted H3PO4 = 0.3666... mol×98 gm/mol=35.9333... gm≈35.93 gm So, option (B) is correct.
(C) nCO2 = 0.5 moles: From the balanced equation, 3 moles of CaCO3 produce 3 moles of CO2. Moles of CO2 produced = 0.5 mol CaCO3×3 mol CaCO33 mol CO2=0.5 mol So, option (C) is correct.
(D) 0.7 mole CO2 is evolved: As calculated above, 0.5 moles of CO2 are evolved, not 0.7 moles. So, option (D) is incorrect.
Conclusion: Options (A), (B), and (C) are correct.
Explanation of the solution:
- Calculate initial moles of reactants using their given masses and molar masses.
- Identify the limiting reagent by comparing the mole ratios of reactants to their stoichiometric coefficients. CaCO3 is the limiting reagent.
- Use the moles of the limiting reagent to calculate the moles and mass of the product Ca3(PO4)2 formed.
- Use the moles of the limiting reagent to calculate the moles of CO2 evolved.
- Use the moles of the limiting reagent to calculate the moles of H3PO4 reacted, then subtract from initial moles to find unreacted amount.
