Question
Question: \(4.5\) moles each of hydrogen and iodine are heated in a sealed 10 liter vessel. At equilibrium 3 m...
4.5 moles each of hydrogen and iodine are heated in a sealed 10 liter vessel. At equilibrium 3 moles of hydrogen iodide was found. The equilibrium constant for
H2(g)+I2(g)⇌2HI(g)
A. 1
B. 10
C. 5
D. 0.33
Solution
The product of the molar concentrations of the products , each raised to the power equal to its stoichiometric Coefficient divided by the product of the molar concentration of the reactants each raised to the power equal to its so accumetric Coefficient is constant at constant temperature and is called equilibrium constant.
Complete step by step answer: It is given that 4.5 moles each of hydrogen and iodine are heated in a sealed 10 liter vessel.
H2(g)+I2(g)⇌2HI(g)
Since, 1 mole of H2 and one mole of I2 combine to give 2 moles of HI then 3 moles of HI would be formed.
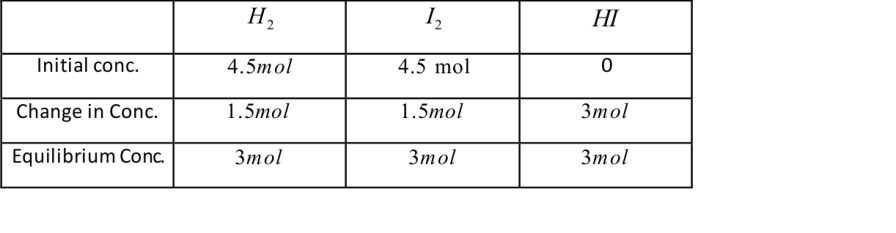
Initially there are 4.5 moles of hydrogen and Iodine gas present in the vessel .It is given that at equilibrium three moles of hydrogen iodide are formed which means there are 3 moles each of hydrogen and Iodine gas left in the container . So we consider the values at equilibrium of both the reactants and products .
Kc =equilibrium constant.
The equilibrium constant for the reaction :-
H2(g)+I2(g)⇌2HI(g)
On substituting the values at equilibrium in the formula of equilibrium constant we get
⇒ Kc=[H2][I2][HI]2=[3][3][3]2=1
So, the correct option is (1)
The value of equilibrium constant is one which indicates that both reactants and products are present at equilibrium.
So, the correct answer is “Option A”.
Note: With the help of the value of equilibrium constant we can predict that in which direction the reaction is moving. For that we first need to find out the concentration quotient or reaction quotient which is the concentration ratio at any stage of the reaction other than the state of chemical equilibrium.
