Question
Question: 100 ml of 0.1 M solution of AB (d = 1.5 gm/ml) is mixed with 100 ml of 0.2 M solution of CB₂ (d = 2....
100 ml of 0.1 M solution of AB (d = 1.5 gm/ml) is mixed with 100 ml of 0.2 M solution of CB₂ (d = 2.5 gm/ml). Calculate the molarity of B− in final solution if the density of final solution is 4 gm/ml. Assuming AB and CB₂ are non reacting & dissociates completely into A+,B−,C+2.
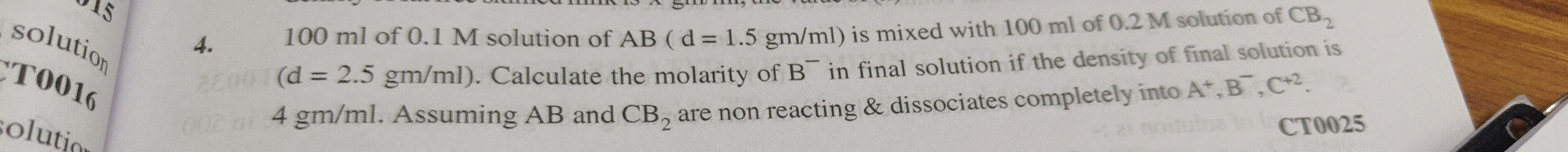
0.5 M
Solution
The problem requires calculating the molarity of B⁻ ions in a final solution formed by mixing two initial solutions, AB and CB₂.
1. Calculate moles of B⁻ from each solution:
-
For AB solution:
- Volume (V₁): 100 ml = 0.1 L
- Molarity (M₁): 0.1 M
- Moles of AB (n_AB) = M₁ × V₁ = 0.1 mol/L × 0.1 L = 0.01 mol
- Since AB dissociates completely (AB→A++B−), moles of B⁻ from AB = 0.01 mol.
-
For CB₂ solution:
- Volume (V₂): 100 ml = 0.1 L
- Molarity (M₂): 0.2 M
- Moles of CB₂ (n_CB₂) = M₂ × V₂ = 0.2 mol/L × 0.1 L = 0.02 mol
- Since CB₂ dissociates completely (CB2→C+2+2B−), moles of B⁻ from CB₂ = 2 × n_CB₂ = 2 × 0.02 mol = 0.04 mol.
2. Calculate total moles of B⁻ in the final solution:
- Total moles of B⁻ (n_B⁻_total) = Moles of B⁻ from AB + Moles of B⁻ from CB₂
- n_B⁻_total = 0.01 mol + 0.04 mol = 0.05 mol
3. Calculate the mass of each initial solution:
- Mass of AB solution (m₁) = Volume₁ × Density₁ = 100 ml × 1.5 gm/ml = 150 gm
- Mass of CB₂ solution (m₂) = Volume₂ × Density₂ = 100 ml × 2.5 gm/ml = 250 gm
4. Calculate the total mass of the final solution:
- Total mass (m_final) = m₁ + m₂ = 150 gm + 250 gm = 400 gm
5. Calculate the total volume of the final solution:
- Density of final solution (d_final) = 4 gm/ml
- Total volume (V_final) = m_final / d_final = 400 gm / 4 gm/ml = 100 ml
- Convert volume to Liters: V_final = 100 ml = 0.1 L
6. Calculate the molarity of B⁻ in the final solution:
- Molarity of B⁻ (M_B⁻) = n_B⁻_total / V_final
- M_B⁻ = 0.05 mol / 0.1 L = 0.5 M
The molarity of B⁻ in the final solution is 0.5 M.
Explanation of the solution:
- Calculate moles of B⁻ from AB (0.1 M×0.1 L=0.01 mol).
- Calculate moles of B⁻ from CB₂ (0.2 M×0.1 L×2=0.04 mol).
- Sum to find total moles of B⁻ (0.01+0.04=0.05 mol).
- Calculate mass of AB solution (100 ml×1.5 gm/ml=150 gm).
- Calculate mass of CB₂ solution (100 ml×2.5 gm/ml=250 gm).
- Sum to find total mass of final solution (150+250=400 gm).
- Calculate total volume of final solution (400 gm/4 gm/ml=100 ml=0.1 L).
- Divide total moles of B⁻ by total volume to find molarity (0.05 mol/0.1 L=0.5 M).
