Question
Question: ...
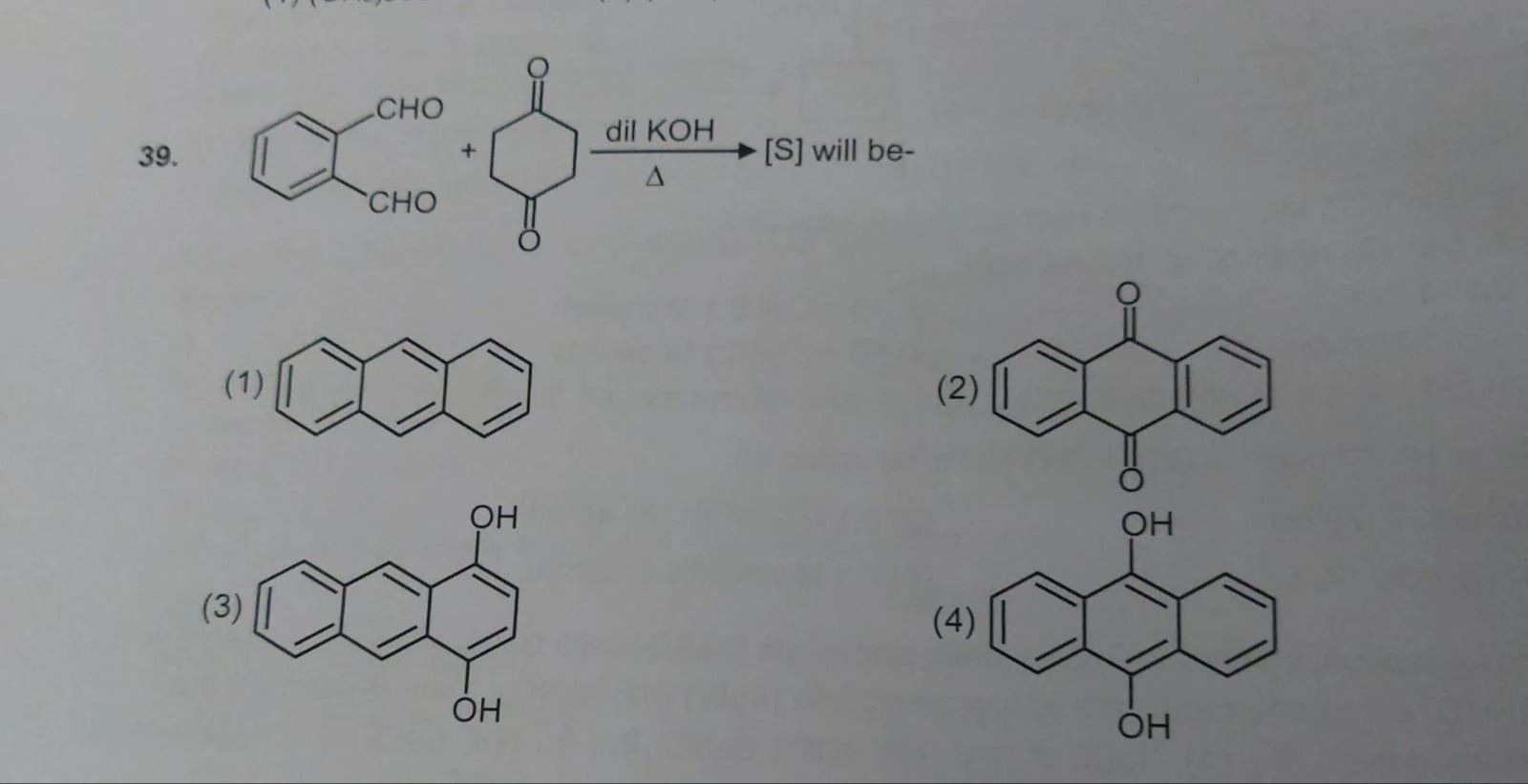
Chemical structure of anthracene, a tricyclic aromatic hydrocarbon consisting of three fused benzene rings in a linear arrangement.
Chemical structure of anthraquinone, a tricyclic aromatic compound consisting of two benzene rings connected by a central six-membered ring with two ketone groups (C=O) at positions 9 and 10.
Chemical structure of 9,10-dihydroxyanthracene, a tricyclic aromatic compound consisting of three fused benzene rings in a linear arrangement, with two hydroxyl groups (OH) attached to the central ring at positions 9 and 10.
Chemical structure of 1,8-dihydroxyanthracene, a tricyclic aromatic compound consisting of three fused benzene rings in a linear arrangement, with two hydroxyl groups (OH) attached to the benzene rings at positions 1 and 8.
Option (2) Anthraquinone
Solution
The reaction involves an ortho‐dialdehyde (benzene with two –CHO groups at adjacent positions, i.e. phthalaldehyde) and a para‐quinone (six‐membered ring with two C=O groups at opposite positions, i.e. p‐benzoquinone) under dilute KOH and heat.
Under these basic, heated conditions, a nucleophilic attack by an enolate generated from the aldehyde onto the quinone occurs, leading to cyclization and subsequent dehydration/oxidation to form a fused tricyclic system containing two carbonyl groups on the central ring.
The resulting structure is that of anthraquinone: three fused benzene rings with ketone groups at the 9 and 10 positions.
Thus, the correct product is anthraquinone.
Core Explanation:
Ortho-dialdehyde reacts with para-benzoquinone in the presence of base and heat to form a tricyclic system which, after cyclization and oxidation, becomes anthraquinone.
