Question
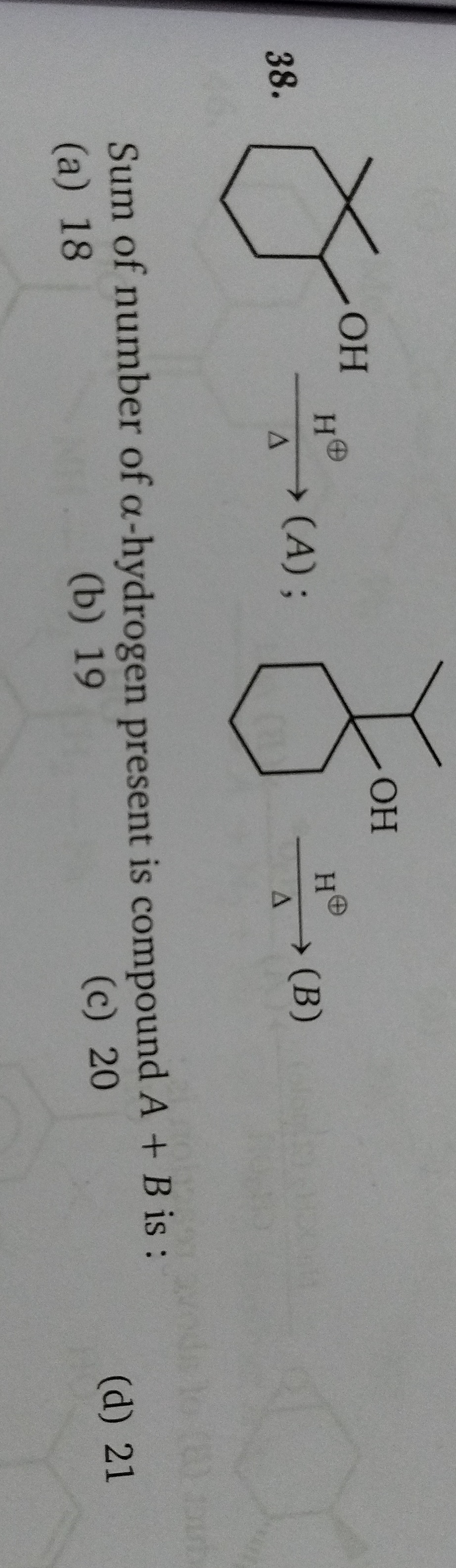
Question: Sum of number of $\alpha$-hydrogen present is compound A + B is:...
Sum of number of α-hydrogen present is compound A + B is:
18
19
20
21
20
Solution
The question asks for the sum of the number of α-hydrogens in compounds A and B, which are the major products of dehydration of 1,1-dimethylcyclohexanol and 1-isopropylcyclohexanol, respectively, under acidic conditions.
First, consider the dehydration of 1,1-dimethylcyclohexanol. The major product A is 1,2-dimethylcyclohexene.
Number of α-hydrogens in A (1,2-dimethylcyclohexene):
CH3 CH3
| |
C=C
/ \
C C
| |
C C
\ /
C
Let's draw the structure with hydrogens.
H3C CH3
| |
C1=C2
/ \
C6 C3
| |
H2C CH2
| |
H2C---CH2
\ /
C4
|
H2C
Allylic hydrogens are on C3, C6, and the methyl carbons.
Hydrogens on C3 (adjacent to C2) = 2. Hydrogens on C6 (adjacent to C1) = 2. Hydrogens on methyl at C1 (adjacent to C1) = 3. Hydrogens on methyl at C2 (adjacent to C2) = 3. Total allylic hydrogens = 2 + 2 + 3 + 3 = 10.
From 1-isopropylcyclohexanol, the major product B is 1-cyclohexylpropene.
CH = C(CH3)2
|
Cyclohexane ring
α-hydrogens are on the carbons adjacent to the double bond. These are the carbons of the ring adjacent to C1 (C2 and C6), and the methyl carbons of the isopropyl group.
Hydrogens on C2 (2) + hydrogens on C6 (2) + hydrogens on methyls (3+3=6). Total = 2 + 2 + 6 = 10.
So, if A is 1,2-dimethylcyclohexene (10 α-hydrogens) and B is 1-cyclohexylpropene (10 α-hydrogens), the sum is 20.
