Question
Question: 2-Phenylbutan-2-ol can not be prepared by...
2-Phenylbutan-2-ol can not be prepared by
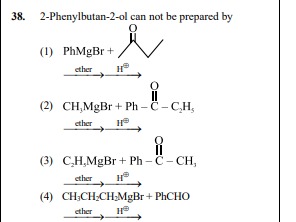
PhMgBr + etherH⊕
CH3MgBr+Ph−∣∣CO−C2H5 etherH⊕
C2H5MgBr+Ph−∣∣CO−CH3 etherH⊕
CH3CH2CH2MgBr+PhCHO etherH⊕
4
Solution
To determine which reaction cannot prepare 2-phenylbutan-2-ol, we first need to understand the structure of 2-phenylbutan-2-ol and the general reaction of Grignard reagents with carbonyl compounds.
The structure of 2-phenylbutan-2-ol is:
CH3CH2−C(OH)(Ph)−CH3
This is a tertiary alcohol. Tertiary alcohols are typically formed by the reaction of a Grignard reagent with a ketone. Aldehydes (except formaldehyde) yield secondary alcohols, and formaldehyde yields primary alcohols.
Let's analyze each option:
-
PhMgBr + Butan-2-one (CH3COCH2CH3)
-
Reactants: Phenylmagnesium bromide (PhMgBr) and Butan-2-one (a ketone).
-
Reaction: The phenyl group (Ph-) from the Grignard reagent attacks the carbonyl carbon of butan-2-one.
CH3COCH2CH3+PhMgBretherCH3C(OMgBr)(Ph)CH2CH3H3O+CH3C(OH)(Ph)CH2CH3
-
Product: 2-phenylbutan-2-ol.
-
This reaction can prepare 2-phenylbutan-2-ol.
-
-
CH3MgBr+Ph−CO−C2H5 (Propiophenone)
-
Reactants: Methylmagnesium bromide (CH3MgBr) and Propiophenone (a ketone).
-
Reaction: The methyl group (CH3−) from the Grignard reagent attacks the carbonyl carbon of propiophenone.
Ph−CO−C2H5+CH3MgBretherPh−C(OMgBr)(CH3)−C2H5H3O+Ph−C(OH)(CH3)−C2H5
-
Product: 2-phenylbutan-2-ol.
-
This reaction can prepare 2-phenylbutan-2-ol.
-
-
C2H5MgBr+Ph−CO−CH3 (Acetophenone)
-
Reactants: Ethylmagnesium bromide (C2H5MgBr) and Acetophenone (a ketone).
-
Reaction: The ethyl group (C2H5−) from the Grignard reagent attacks the carbonyl carbon of acetophenone.
Ph−CO−CH3+C2H5MgBretherPh−C(OMgBr)(C2H5)−CH3H3O+Ph−C(OH)(C2H5)−CH3
-
Product: 2-phenylbutan-2-ol.
-
This reaction can prepare 2-phenylbutan-2-ol.
-
-
CH3CH2CH2MgBr+PhCHO (Benzaldehyde)
-
Reactants: n-Propylmagnesium bromide (CH3CH2CH2MgBr) and Benzaldehyde (an aldehyde).
-
Reaction: The n-propyl group (CH3CH2CH2−) from the Grignard reagent attacks the carbonyl carbon of benzaldehyde.
PhCHO+CH3CH2CH2MgBretherPh−CH(OMgBr)−CH2CH2CH3H3O+Ph−CH(OH)−CH2CH2CH3
-
Product: 1-phenylbutan-1-ol. This is a secondary alcohol, not 2-phenylbutan-2-ol.
-
This reaction cannot prepare 2-phenylbutan-2-ol.
-
Therefore, the reaction that cannot prepare 2-phenylbutan-2-ol is option (4).
