Question
Question: When methane is burnt completely, oxidation state of carbon changes from...
When methane is burnt completely, oxidation state of carbon changes from
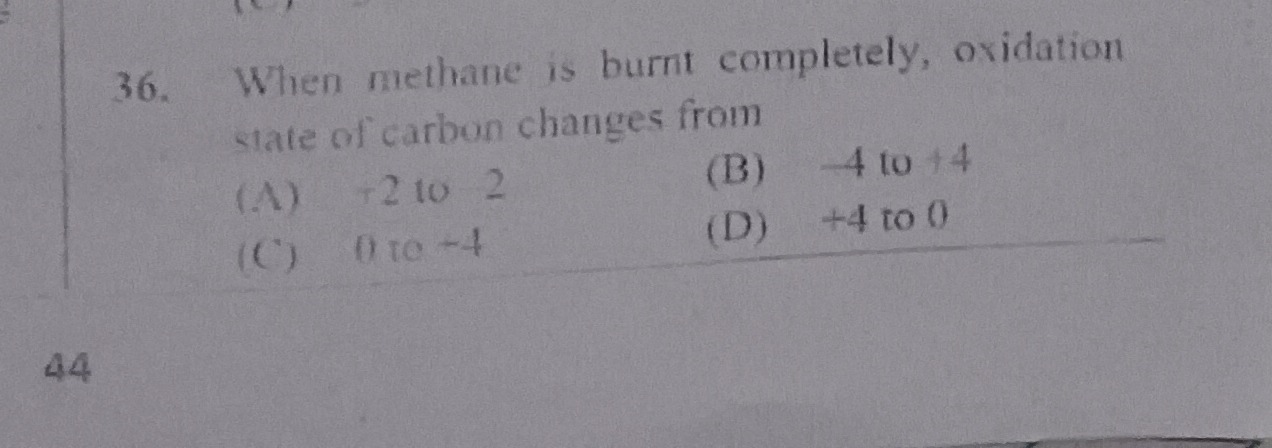
A
+2 to 2
B
-4 to +4
C
0 to -4
D
+4 to 0
Answer
Option (B)
Explanation
Solution
For methane, CH4:
x+4(+1)=0⇒x=−4.For carbon dioxide, CO2:
x+2(−2)=0⇒x=+4.Thus, the oxidation state of carbon changes from -4 to +4.
In CH4, carbon’s oxidation state is -4; in CO2, it is +4.
