Question
Question: The frequency of revolution of electron in n$^{th}$ Bohr orbit is $v_n$. The graph between log n and...
The frequency of revolution of electron in nth Bohr orbit is vn. The graph between log n and log (vn/v1) may be
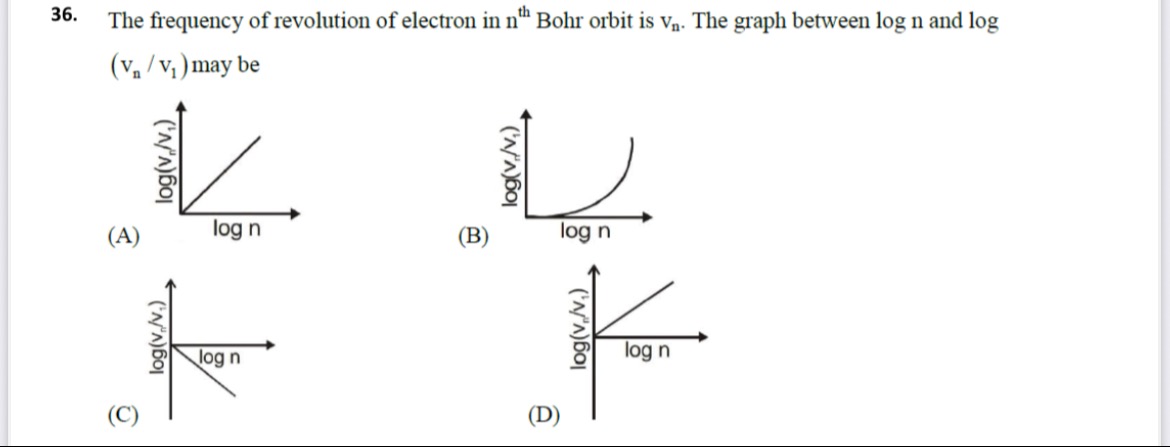
(A)
(B)
(C)
(D)
C
Solution
The frequency of revolution of an electron in the nth Bohr orbit, denoted as vn, is given by the formula:
vn=circumference of n-th orbitvelocity of electron in n-th orbit=2πrnvn
We know the expressions for the velocity (vn) and radius (rn) of an electron in the nth Bohr orbit for a hydrogen-like atom (atomic number Z):
-
Velocity: vn=2ϵ0nhZe2
This shows vn∝nZ. For a given atom (Z is constant), vn∝n1.
-
Radius: rn=πmeZe2n2h2ϵ0
This shows rn∝Zn2. For a given atom (Z is constant), rn∝n2.
Now, substitute these proportionalities into the frequency formula:
vn∝n21/n∝n31
So, the frequency of revolution vn is inversely proportional to the cube of the principal quantum number n. We can write this as:
vn=Kn31
where K is a constant of proportionality.
For the first Bohr orbit (n=1), the frequency of revolution v1 is:
v1=K131=K
Now, let's find the ratio vn/v1:
v1vn=KK/n3=n31
We need to find the graph between logn and log(vn/v1). Let's take the logarithm of both sides of the ratio equation:
log(v1vn)=log(n31)
Using logarithm properties, log(1/x)=−log(x) and log(xy)=ylog(x):
log(v1vn)=log(n−3)
log(v1vn)=−3logn
Let Y=log(v1vn) and X=logn.
The equation becomes:
Y=−3X
This is the equation of a straight line passing through the origin (since Y=0 when X=0) with a negative slope (-3).
Comparing this with the given graphs:
(A) Shows a straight line with a positive slope passing through the origin.
(B) Shows a curve.
(C) Shows a straight line with a negative slope passing through the origin.
(D) Shows a straight line with a positive slope not passing through the origin.
Therefore, graph (C) correctly represents the relationship between logn and log(vn/v1).
