Question
Question: Identify the pair of complex which are stereoisomer of each other -...
Identify the pair of complex which are stereoisomer of each other -
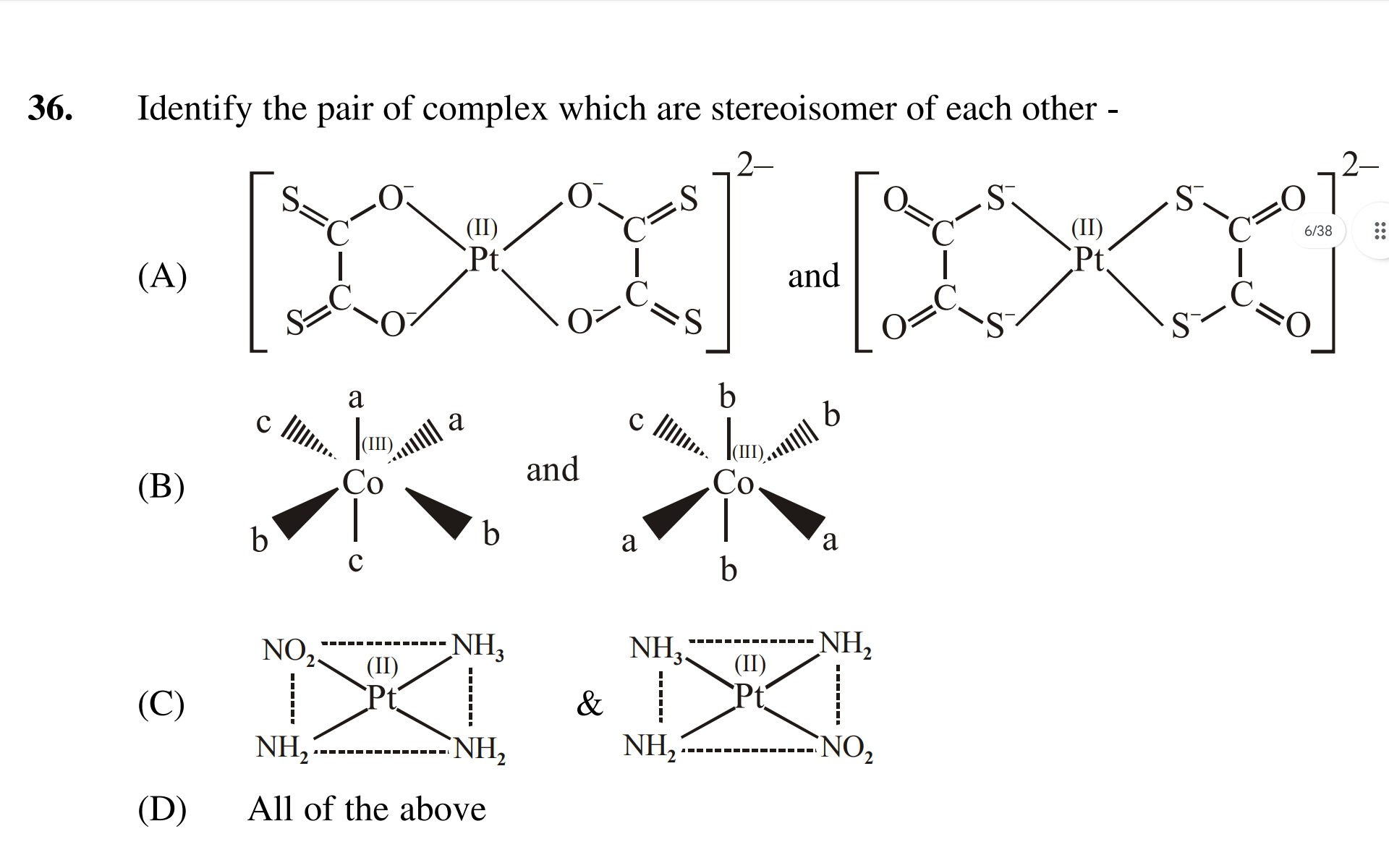
and
and
&
All of the above
All of the above
Solution
Stereoisomers are compounds with the same molecular formula and connectivity but different spatial arrangements of atoms.
Option (A): The complexes are square planar. The first complex shows two dithiooxalate ligands coordinated through sulfur atoms in a cis arrangement. The second complex also shows two dithiooxalate ligands coordinated through sulfur atoms in a trans arrangement. Cis and trans isomers are geometric stereoisomers. Thus, the pair in (A) are stereoisomers.
Option (B): The complexes are octahedral with the general formula [Co(a)2(b)2(c)2]. The first complex shows the two 'c' ligands trans to each other, and the 'a' and 'b' ligands in cis arrangements within the equatorial plane. The second complex shows one 'c' and one 'b' ligand trans to each other, with the 'a' and remaining 'b' ligands in trans arrangements within the equatorial plane. These represent different geometric isomers of the same complex, hence they are stereoisomers.
Option (C): The complexes are square planar platinum(II) complexes. Assuming the intended representation, the first complex shows ligands arranged in a manner that suggests a specific isomer, while the second complex shows a different spatial arrangement of the same ligands. Despite the potential inaccuracies in the drawing regarding the number of ligands, if interpreted as two distinct geometric isomers of a square planar complex (e.g., different arrangements of cis and trans ligands), they would be stereoisomers. Given that options (A) and (B) are clearly stereoisomers, and option (D) is "All of the above," it implies that option (C) is also intended to represent stereoisomers.
Therefore, all pairs presented are stereoisomers of each other.
