Question
Question: In given reaction sequence. (2)...
In given reaction sequence.
(2)
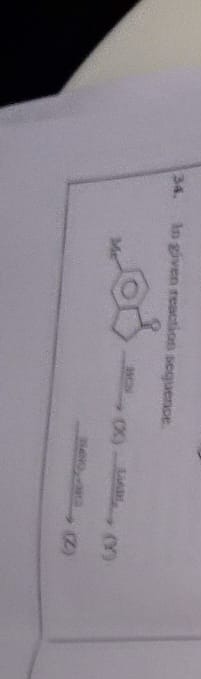
Y = 5-methyl-2,3-dihydro-1H-inden-1-ol
Solution
The given reaction sequence involves a starting material, 5-methyl-1-indanone, which undergoes two consecutive reactions.
Step 1: Reaction of 5-methyl-1-indanone with NaBH4
-
Starting Material: 5-methyl-1-indanone is a cyclic ketone.
-
Reagent: NaBH4 (Sodium borohydride) is a mild reducing agent that selectively reduces aldehydes and ketones to alcohols.
-
Product (X): The ketone group (C=O) in 5-methyl-1-indanone will be reduced to a secondary alcohol (CH-OH). The methyl group and the aromatic ring remain unaffected. Therefore, product (X) is 5-methyl-2,3-dihydro-1H-inden-1-ol (or 5-methyl-1-indanol).
Step 2: Reaction of (X) with MeMgBr followed by H3O+
-
Starting Material: (X) is 5-methyl-2,3-dihydro-1H-inden-1-ol, a secondary alcohol.
-
Reagent: MeMgBr (Methylmagnesium bromide) is a Grignard reagent. Grignard reagents are very strong bases and strong nucleophiles.
-
Reaction: When a Grignard reagent reacts with an alcohol, it acts as a strong base and deprotonates the acidic hydroxyl proton. R-OH + CH3MgBr → R-O-MgBr + CH4 (methane gas is evolved) This reaction forms an alkoxide (R-O-MgBr).
-
Workup: The subsequent acid workup (H3O+) protonates the alkoxide to regenerate the alcohol. R-O-MgBr + H3O+ → R-OH + Mg(OH)Br (or other magnesium salts)
-
Product (Y): Based on the standard reactivity of Grignard reagents with alcohols, the alcohol (X) will be regenerated after the deprotonation and subsequent acid workup. This means that product (Y) would be identical to product (X).
Conclusion:
Following the reaction sequence strictly as written, the second step results in the regeneration of the alcohol (X), making (Y) identical to (X). While this might seem like a trivial step in a multi-step synthesis problem, it is the chemically accurate outcome based on the provided reagents.
The question asks for the product of the given reaction sequence. Assuming the question follows the sequence literally, the product (Y) is the same as (X).
The final answer is Y=5−methyl−2,3−dihydro−1H−inden−1−ol
Explanation of the solution:
- Step 1 (Ketone to Alcohol): 5-methyl-1-indanone (a ketone) is reduced by NaBH4 to 5-methyl-1-indanol (a secondary alcohol), which is compound (X). NaBH4 is a selective reducing agent for carbonyls.
- Step 2 (Alcohol with Grignard): Compound (X), a secondary alcohol, reacts with MeMgBr. Grignard reagents are strong bases and deprotonate alcohols. The resulting alkoxide, upon acid workup (H3O+), regenerates the original alcohol. Thus, compound (Y) is identical to compound (X).
Answer:
The final product (Y) is 5-methyl-2,3-dihydro-1H-inden-1-ol.
