Question
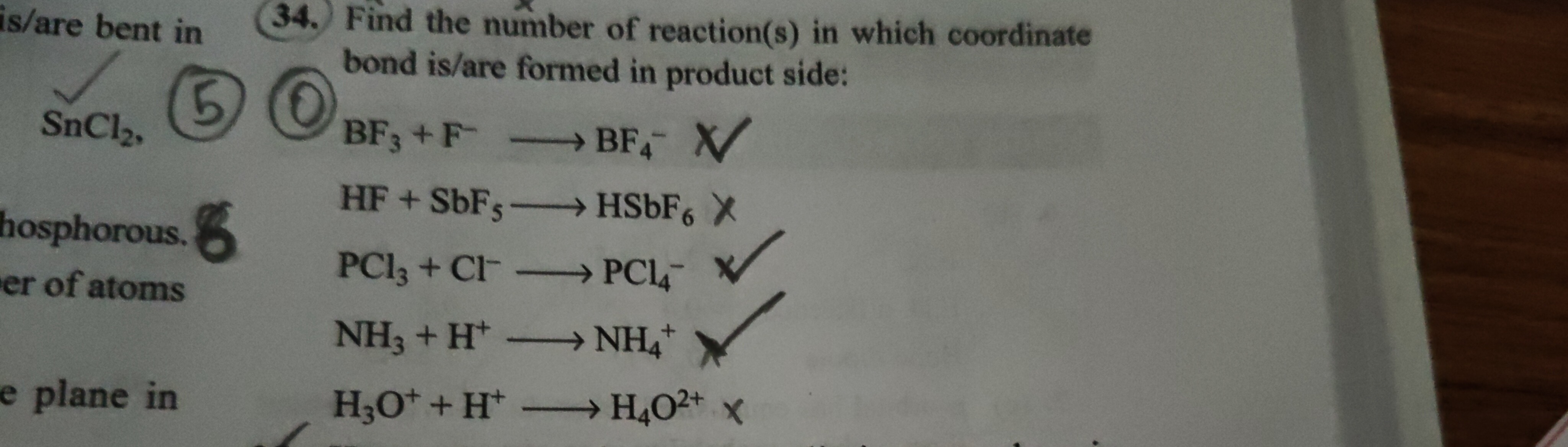
Question: Find the number of reaction(s) in which coordinate bond is/are formed in product side: $BF_3 + F^- ...
Find the number of reaction(s) in which coordinate bond is/are formed in product side:
BF3+F−⟶BF4− X
HF+SbF5⟶HSbF6 X
PCl3+Cl−⟶PCl4−
NH3+H+⟶NH4+
H3O++H+⟶H4O2+ X
Answer
4
Explanation
Solution
A coordinate bond (or dative bond) is a type of covalent bond where both shared electrons are contributed by one atom (the donor) to another atom (the acceptor). This typically occurs in Lewis acid-base reactions.
Let's analyze each reaction:
-
BF3+F−⟶BF4−
- BF3 (Boron trifluoride) is a Lewis acid because boron has an incomplete octet (6 valence electrons) and an empty p-orbital.
- F− (Fluoride ion) is a Lewis base because it has lone pairs of electrons to donate.
- In BF4−, the fluoride ion donates a lone pair of electrons to the empty p-orbital of boron, forming a B-F bond. This is a coordinate bond.
- Yes, a coordinate bond is formed.
-
HF+SbF5⟶HSbF6
- This reaction involves the formation of a superacid, fluoroantimonic acid. The reaction can be viewed as HF acting as a source of F− and SbF5 acting as a strong Lewis acid.
- SbF5 accepts a fluoride ion (F−) to form SbF6−. In this process, a lone pair from the fluoride ion is donated to an empty orbital of antimony.
- The product HSbF6 is an ionic compound, (H+)(SbF6−). The bond between Sb and the incoming F in SbF6− is a coordinate bond.
- Yes, a coordinate bond is formed.
-
PCl3+Cl−⟶PCl4−
- PCl3 (Phosphorus trichloride) has a lone pair on phosphorus and also empty d-orbitals, allowing it to act as a Lewis acid by expanding its octet.
- Cl− (Chloride ion) is a Lewis base, donating a lone pair.
- In PCl4−, the chloride ion donates a lone pair to an empty d-orbital of phosphorus, forming a P-Cl bond. This is a coordinate bond.
- Yes, a coordinate bond is formed.
-
NH3+H+⟶NH4+
- NH3 (Ammonia) is a Lewis base because nitrogen has a lone pair of electrons.
- H+ (Proton) is a Lewis acid because it has an empty 1s orbital.
- In NH4+ (Ammonium ion), the lone pair on the nitrogen atom is donated to the empty 1s orbital of the proton, forming an N-H bond. This is a coordinate bond.
- Yes, a coordinate bond is formed.
-
H3O++H+⟶H4O2+
- H3O+ (Hydronium ion) has one lone pair on the oxygen atom. If it were to accept another H+, it would form H4O2+.
- The species H4O2+ would have oxygen bonded to four hydrogen atoms with a formal charge of +2 on oxygen. This species is highly unstable and is not observed under normal chemical conditions.
- The product given, H4O2+, is also chemically unusual and does not correspond to a stable, commonly recognized species formed from H3O+ and H+.
- Therefore, this reaction does not lead to the formation of a stable product via a coordinate bond.
- No, a coordinate bond is not formed in a stable product.
Based on the analysis, a coordinate bond is formed in the product side for the first four reactions.
Explanation of the solution:
A coordinate bond forms when one atom donates both electrons for a shared pair.
- BF3+F−⟶BF4−: F− donates a lone pair to electron-deficient B in BF3. Coordinate B-F bond forms.
- HF+SbF5⟶HSbF6: F− from HF donates a lone pair to Lewis acidic SbF5 to form SbF6−. Coordinate Sb-F bond forms.
- PCl3+Cl−⟶PCl4−: Cl− donates a lone pair to P in PCl3 (using P's empty d-orbitals). Coordinate P-Cl bond forms.
- NH3+H+⟶NH4+: Lone pair on N in NH3 is donated to H+. Coordinate N-H bond forms.
- H3O++H+⟶H4O2+: This reaction is chemically unsound. H3O+ accepting another H+ would lead to highly unstable H4O2+, not H4O2+. No stable coordinate bond is formed.
Thus, 4 reactions involve coordinate bond formation.
