Question
Question: For the reaction Product B is : ...
For the reaction
Product B is :
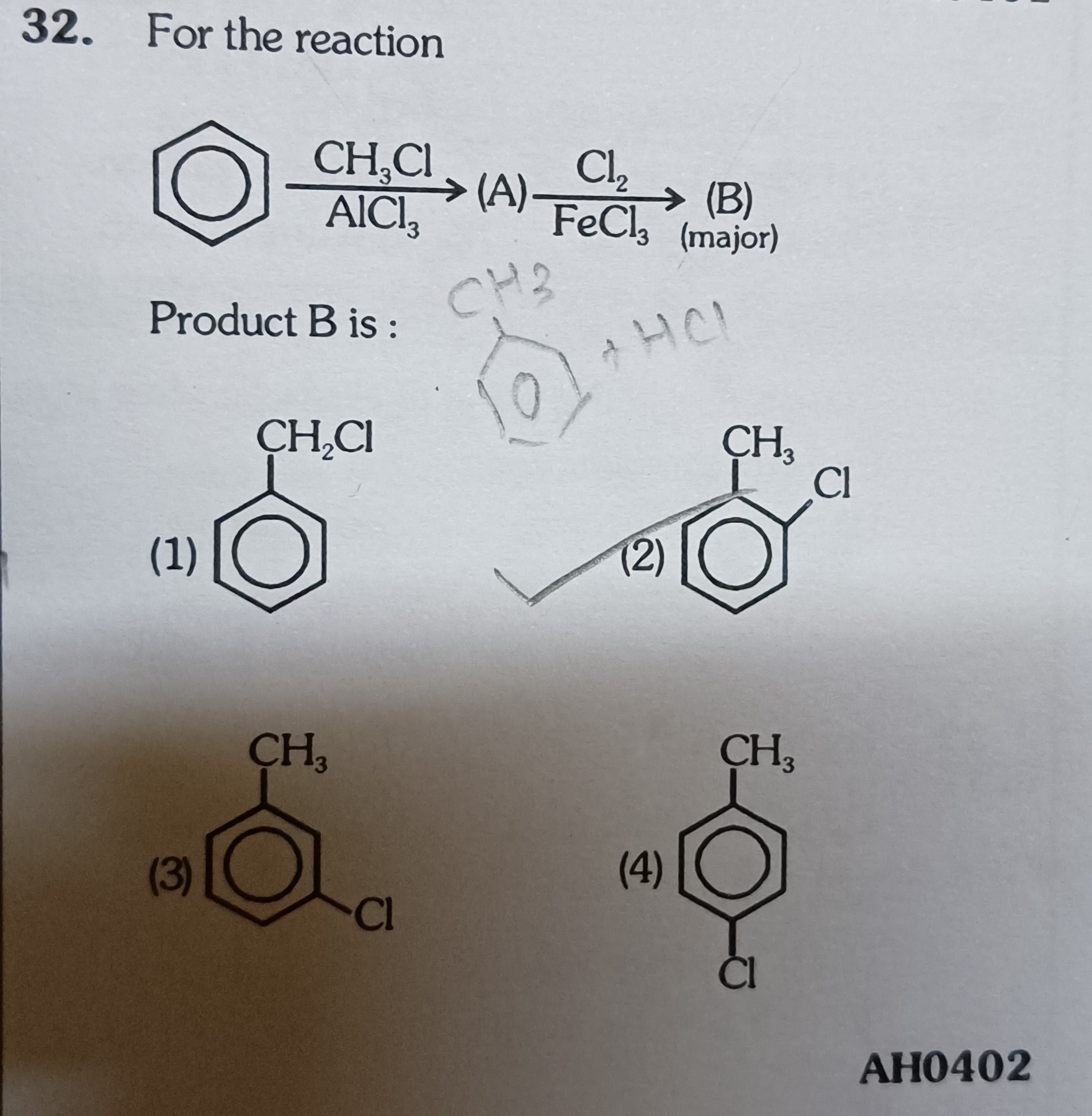
:c1ccccc1CCl (Benzyl chloride)
:c1ccccc1(C)Cl (2-chlorotoluene or o-chlorotoluene)
:c1ccccc1(C)C(Cl)=C (Incorrect structure, likely intended as 3-chlorotoluene or m-chlorotoluene)
:c1ccc(C)cc1Cl (4-chlorotoluene or p-chlorotoluene)
4-chlorotoluene
Solution
The reaction proceeds in two steps:
Step 1: Friedel-Crafts Alkylation
Benzene reacts with methyl chloride (CH3Cl) in the presence of anhydrous aluminium chloride (AlCl3). This is a Friedel-Crafts alkylation reaction, which is an electrophilic aromatic substitution.
The electrophile generated is the methyl carbocation (CH3+).
Benzene is alkylated to form toluene (methylbenzene).
Product (A) is Toluene: :c1ccccc1C
Step 2: Electrophilic Aromatic Substitution (Chlorination)
Toluene (Product A) reacts with chlorine (Cl2) in the presence of ferric chloride (FeCl3). This is an electrophilic aromatic substitution reaction (halogenation).
The methyl group (-CH3) on the benzene ring is an activating group and an ortho-para director. This means it activates the benzene ring towards electrophilic substitution and directs the incoming electrophile (in this case, Cl+) to the ortho and para positions relative to itself.
Among ortho and para isomers, the para isomer is generally the major product due to less steric hindrance between the existing methyl group and the incoming chlorine atom.
Therefore, Product (B) (major product) is 4-chlorotoluene.
