Question
Question: Find the value of z in H₄P₂O₈. z = $\frac{x+d}{a+c+b}$ Total number of σ bond = x Total number of ...
Find the value of z in H₄P₂O₈.
z = a+c+bx+d
Total number of σ bond = x Total number of lone pair of e⁻s are = a Total number of sp³ hybridised atoms are b Total number of pπ-pπ bonds are c Total number of pπ-dπ bonds are d
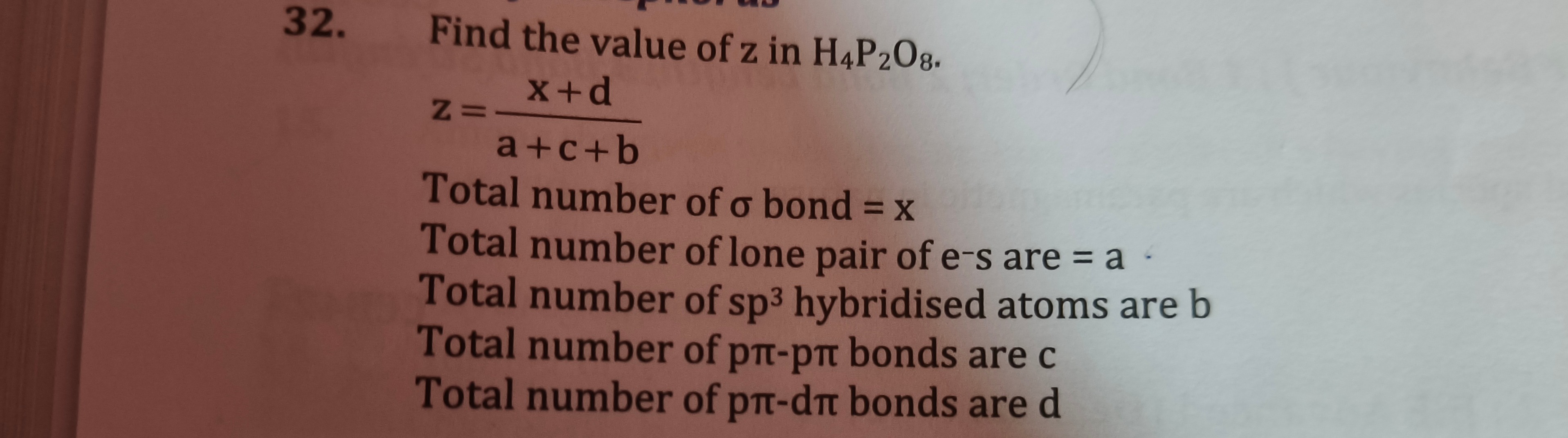
z = 85
Solution
To find the value of z, we first need to determine the structure of H₄P₂O₈ and then calculate the values of x, a, b, c, and d.
1. Determine the structure of H₄P₂O₈: First, calculate the oxidation state of Phosphorus (P) in H₄P₂O₈. Let the oxidation state of P be S. 4(+1) + 2(S) + 8(-2) = 0 4 + 2S - 16 = 0 2S = 12 S = +6 Since the maximum oxidation state of Phosphorus is +5, an oxidation state of +6 indicates the presence of a peroxide (O-O) linkage in the molecule. Thus, H₄P₂O₈ is peroxodiphosphoric acid. Its structure is:
Simplified structural representation:
O O
|| ||
HO -- P -- O -- O -- P -- OH
| |
OH OH
2. Calculate x (Total number of σ bonds):
- P=O bonds: 2 (each has 1 σ bond) = 2 σ bonds
- P-OH bonds: 4 (each has 1 σ bond) = 4 σ bonds
- P-O (in P-O-O linkage) bonds: 2 (each has 1 σ bond) = 2 σ bonds
- O-O bond: 1 (1 σ bond) = 1 σ bond
- O-H bonds: 4 (each has 1 σ bond) = 4 σ bonds
Total σ bonds (x) = 2 + 4 + 2 + 1 + 4 = 13. So, x = 13.
3. Calculate a (Total number of lone pairs of electrons):
- Phosphorus (P) atoms: Each P atom is in a +5 oxidation state and forms 5 bonds (1 double, 3 single). All 5 valence electrons are involved in bonding, so no lone pairs on P atoms.
- Oxygen (O) atoms: There are 8 oxygen atoms in total.
- Two P=O oxygen atoms: Each forms one double bond (2 bonds). With 6 valence electrons, 2 are used in bonding, leaving 4 non-bonding electrons (2 lone pairs) on each. (2 O atoms * 2 lone pairs/O = 4 lone pairs)
- Four P-OH oxygen atoms: Each forms two single bonds (one to P, one to H). With 6 valence electrons, 2 are used in bonding, leaving 4 non-bonding electrons (2 lone pairs) on each. (4 O atoms * 2 lone pairs/O = 8 lone pairs)
- Two P-O-O oxygen atoms: Each forms two single bonds (one to P, one to O). With 6 valence electrons, 2 are used in bonding, leaving 4 non-bonding electrons (2 lone pairs) on each. (2 O atoms * 2 lone pairs/O = 4 lone pairs)
Total lone pairs (a) = 4 + 8 + 4 = 16. So, a = 16.
4. Calculate b (Total number of sp³ hybridised atoms): Hybridization is determined by the steric number (number of sigma bonds + number of lone pairs).
- Phosphorus (P) atoms: Each P atom forms 4 sigma bonds (1 from P=O, 3 from P-O single bonds) and has 0 lone pairs. Steric number = 4 + 0 = 4. Hence, each P atom is sp³ hybridized. (2 P atoms * 1 sp³ /P = 2 sp³ atoms)
- Oxygen (O) atoms:
- Two P=O oxygen atoms: Each forms 1 sigma bond and has 2 lone pairs. Steric number = 1 + 2 = 3. Hence, sp² hybridized. (Not sp³)
- Four P-OH oxygen atoms: Each forms 2 sigma bonds and has 2 lone pairs. Steric number = 2 + 2 = 4. Hence, sp³ hybridized. (4 O atoms * 1 sp³ /O = 4 sp³ atoms)
- Two P-O-O oxygen atoms: Each forms 2 sigma bonds and has 2 lone pairs. Steric number = 2 + 2 = 4. Hence, sp³ hybridized. (2 O atoms * 1 sp³ /O = 2 sp³ atoms)
Total sp³ hybridised atoms (b) = 2 (from P) + 4 (from P-OH O) + 2 (from P-O-O O) = 8. So, b = 8.
5. Calculate c (Total number of pπ-pπ bonds): In oxoacids of phosphorus, the π-bond in P=O is formed by the overlap between the 2p orbital of oxygen and the vacant 3d orbital of phosphorus. This is a pπ-dπ bond. There are no other atoms forming pπ-pπ bonds. So, c = 0.
6. Calculate d (Total number of pπ-dπ bonds): There are two P=O double bonds in the molecule. Each P=O double bond consists of one σ bond and one pπ-dπ bond. So, d = 2.
7. Calculate z: z = a+c+bx+d Substitute the calculated values: z = 16+0+813+2 z = 2415 Simplify the fraction by dividing both numerator and denominator by their greatest common divisor, which is 3: z = 24÷315÷3 z = 85
The final answer is 85.
Explanation of the solution: The molecule H₄P₂O₈ is peroxodiphosphoric acid, confirmed by the +6 oxidation state of P, indicating an O-O peroxide linkage.
- σ bonds (x): Count all single bonds and the sigma component of double bonds. (2 P=O + 4 P-OH + 2 P-O + 1 O-O + 4 O-H = 13).
- Lone pairs (a): P has no lone pairs. Each O atom (P=O, P-OH, P-O-O) has 2 lone pairs. (22 + 42 + 2*2 = 16).
- sp³ atoms (b): Each P is sp³ (4 sigma bonds). P-OH and P-O-O oxygens are sp³ (2 sigma bonds, 2 lone pairs). P=O oxygens are sp². (2 P + 4 P-OH O + 2 P-O-O O = 8).
- pπ-pπ bonds (c): None, as P=O involves pπ-dπ bonding.
- pπ-dπ bonds (d): Two P=O bonds, each contributing one pπ-dπ bond.
- Calculate z: Substitute values into the given formula: z = (13+2)/(16+0+8) = 15/24 = 5/8.
