Question
Question: $CH_2=CHCH_2CH=CH_2 \xrightarrow{NBS} X \text{ (Major), (X) is:}$ ...
CH2=CHCH2CH=CH2NBSX (Major), (X) is:
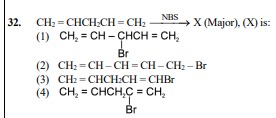
CH2=CH−Br∣CHCH=CH2
CH2=CH−CH=CH−CH2−Br
CH2=CHCH2CH=CHBr
CH2=CHCH2Br∣C=CH2
CH2=CH−CH=CH−CH2−Br
Solution
The reaction involves the allylic bromination of 1,4-pentadiene (CH2=CH−CH2−CH=CH2) using N-bromosuccinimide (NBS). NBS selectively brominates at allylic positions via a free radical mechanism.
1. Formation of Allylic Radical:
The allylic hydrogens are located on the carbon atom adjacent to a double bond. In 1,4-pentadiene, the central methylene group (CH2 at C3) has allylic hydrogens. Abstraction of a hydrogen atom from C3 by a bromine radical (Br⋅) leads to the formation of a resonance-stabilized allylic radical (pentadienyl radical):
CH2=CH−CH2−CH=CH2−H⋅CH2=CH−C˙H−CH=CH2
This radical can exist in multiple resonance forms:
(I) CH2=CH−C˙H−CH=CH2 (Radical at C3)
(II) C˙H2−CH=CH−CH=CH2 (Radical at C1, by shifting the π bond from C1-C2 to C2-C3)
(III) CH2=CH−CH=CH−C˙H2 (Radical at C5, by shifting the π bond from C4-C5 to C3-C4)
Note that (II) and (III) are equivalent by symmetry.
2. Bromination of the Allylic Radical:
The bromine atom can add to any carbon atom bearing significant radical character (C1, C3, or C5).
-
Addition at C3:
From resonance form (I), addition of Br⋅ at C3 gives:
CH2=CH−Br∣CH−CH=CH2
This product is 3-bromo-1,4-pentadiene. It contains two isolated double bonds.
-
Addition at C1 (or C5):
From resonance form (II) or (III), addition of Br⋅ at C1 (or C5) gives:
Br−CH2−CH=CH−CH=CH2 (or CH2=CH−CH=CH−CH2−Br)
This product is 1-bromo-1,3-pentadiene. It contains two conjugated double bonds.
3. Determining the Major Product:
In radical reactions involving resonance-stabilized intermediates, the major product is often the one that leads to the most stable final product.
Comparing the two possible products:
- 3-bromo-1,4-pentadiene has isolated double bonds.
- 1-bromo-1,3-pentadiene has conjugated double bonds.
Conjugated dienes are significantly more stable than isolated dienes due to the delocalization of π electrons. Therefore, 1-bromo-1,3-pentadiene is the thermodynamically more stable product and will be the major product.
