Question
Question: Rank the following substances in order of decreasing heat of combustion (maximum $\rightarrow$ minim...
Rank the following substances in order of decreasing heat of combustion (maximum → minimum).
- 1,1-dimethylcyclopentane
- 1,1-dimethylcyclobutane
- Cyclohexane
- 1,1-dimethylcyclopropane
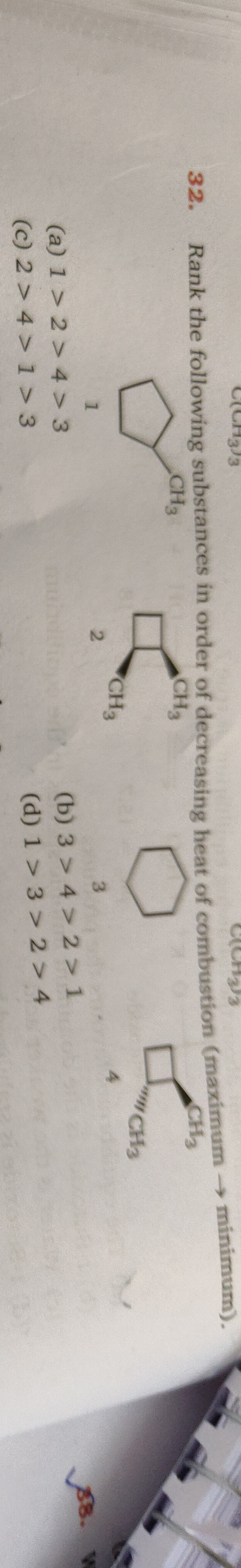
A
1 > 2 > 4 > 3
B
3 > 4 > 2 > 1
C
2 > 4 > 1 > 3
D
1 > 3 > 2 > 4
Answer
1 > 2 > 4 > 3
Explanation
Solution
The heat of combustion is primarily determined by the number of carbon atoms and the stability of the molecule. Heat of combustion increases with the number of carbon atoms and decreases with molecular stability. Ring strain reduces molecular stability, thus increasing the heat of combustion.
- 1,1-dimethylcyclopentane (C7H14): Has the most carbon atoms (7) and low ring strain associated with the cyclopentane ring. This combination leads to the highest heat of combustion.
- 1,1-dimethylcyclobutane (C6H12): Has 6 carbon atoms and moderate ring strain from the cyclobutane ring. Its heat of combustion is expected to be high due to the number of carbons, but less than molecule 1.
- Cyclohexane (C6H12): Has 6 carbon atoms but is a stable, unstrained ring system. Compared to 1,1-dimethylcyclobutane (molecule 2), it is more stable and thus has a lower heat of combustion.
- 1,1-dimethylcyclopropane (C5H10): Has fewer carbon atoms (5) but exhibits very high ring strain due to the cyclopropane ring. This significant strain energy contributes substantially to its heat of combustion, making it higher than cyclohexane (molecule 3) despite having fewer carbons.
Considering these factors, the order of decreasing heat of combustion is: 1 (most carbons, low strain) > 2 (6 carbons, moderate strain) > 4 (fewer carbons, high strain) > 3 (6 carbons, no strain).
Therefore, the ranking is 1 > 2 > 4 > 3.
