Question
Question: 3.15 g oxalic acid $[(COOH)_2.xH_2O]$ was dissolved to make 500 mL solution. On titration, 33.36 mL ...
3.15 g oxalic acid [(COOH)2.xH2O] was dissolved to make 500 mL solution. On titration, 33.36 mL of this solution were neutralised by 50 mL N/15 NaOH. The value of x will be
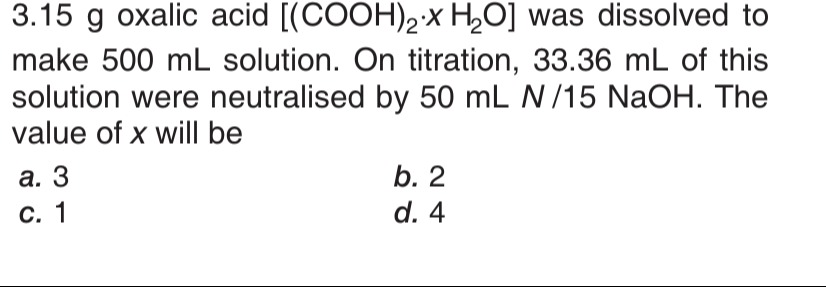
3
2
1
4
2
Solution
The reaction between oxalic acid [(COOH)2.xH2O] and NaOH is a neutralization reaction. Oxalic acid is a dibasic acid, so its n-factor is 2.
The normality of the NaOH solution is given as N/15, which means 1/15 N. The volume of NaOH solution used is 50 mL.
The milliequivalents (meq) of NaOH used in the titration are: meq of NaOH = Normality of NaOH × Volume of NaOH (in mL) meq of NaOH = (1/15N)×(50mL)=50/15meq=10/3meq.
At the equivalence point of the titration, the milliequivalents of oxalic acid in the titrated volume are equal to the milliequivalents of NaOH used. The volume of oxalic acid solution titrated was 33.36 mL. meq of oxalic acid in 33.36 mL = 10/3meq.
The normality of the oxalic acid solution is: Normality of oxalic acid = Volume of oxalic acid solution (in mL)meq of oxalic acid=33.36mL10/3meq.
The total volume of the oxalic acid solution prepared was 500 mL. The total milliequivalents of oxalic acid in the 500 mL solution are: Total meq of oxalic acid = Normality of oxalic acid × Total volume of solution (in mL) Total meq of oxalic acid = (33.3610/3)×500meq.
The initial mass of hydrated oxalic acid [(COOH)2.xH2O] dissolved to make the 500 mL solution was 3.15 g. The relationship between mass, milliequivalents, and equivalent weight is: Total meq = Equivalent weight (in g/eq)Mass (in g)×1000 So, Equivalent weight = Total meqMass (in g)×1000 Equivalent weight of [(COOH)2.xH2O] = (33.3610/3)×500meq3.15g×1000 Equivalent weight = 10×5003.15×1000×33.36×3=50003.15×1000×33.36×3 Equivalent weight = 53.15×33.36×3=5315.252=63.0504g/eq.
The molar mass of anhydrous oxalic acid (COOH)2 is approximately 2×(12+2×16+1)=2×45=90 g/mol. The molar mass of water H2O is approximately 2×1+16=18 g/mol. The molar mass of hydrated oxalic acid [(COOH)2.xH2O] is approximately (90+18x) g/mol. The equivalent weight of oxalic acid is its molar mass divided by its n-factor (which is 2). Equivalent weight = 2Molar mass=290+18x.
Equating the calculated equivalent weight from the titration data to the expression in terms of x: 290+18x=63.0504 90+18x=2×63.0504=126.1008 18x=126.1008−90=36.1008 x=1836.1008≈2.0056.
Since x must be an integer representing the number of water molecules of hydration, the value of x is approximately 2. The slight deviation from exactly 2 is likely due to experimental variations or rounding in the provided data. Oxalic acid commonly crystallizes as the dihydrate.
Assuming x = 2, the equivalent weight would be (90+18×2)/2=(90+36)/2=126/2=63. If the equivalent weight is exactly 63, the total meq in 500 mL is (3.15/63)×1000=0.05×1000=50 meq. The normality of the solution is 50meq/500mL=0.1N. The meq in 33.36 mL is 0.1N×33.36mL=3.336meq. The meq of NaOH used was 10/3=3.333...meq. The values 3.336 and 3.333... are very close, confirming that x=2 is the correct value.
