Question
Question: Which has the highest boiling point?...
Which has the highest boiling point?
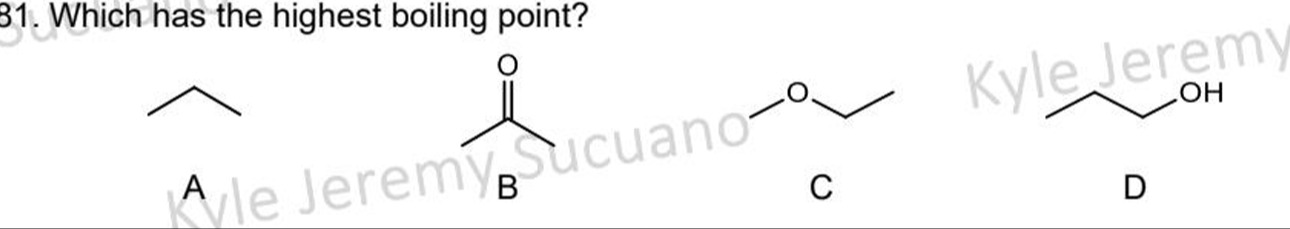
Propane
Acetone
Methoxyethane
1-butanol
D
Solution
Compound A is propane (C₃H₈), with a molecular weight of 44 g/mol. It's a nonpolar alkane, primarily exhibiting London dispersion forces (LDF).
Compound B is acetone (C₃H₆O), with a molecular weight of 58 g/mol. It's a polar ketone, showing LDF and dipole-dipole interactions due to the carbonyl group.
Compound C is methoxyethane (C₃H₈O), with a molecular weight of 60 g/mol. It's a polar ether, exhibiting LDF and dipole-dipole interactions due to the C-O bond.
Compound D is 1-butanol (C₄H₁₀O), with a molecular weight of 74 g/mol. It's a polar alcohol, capable of LDF, dipole-dipole interactions, and hydrogen bonding due to the -OH group.
Boiling point is directly influenced by the strength of intermolecular forces. Hydrogen bonding is considerably stronger than dipole-dipole interactions and LDF. Consequently, the compound with the most robust intermolecular forces will possess the highest boiling point. As 1-butanol (D) is the sole compound capable of forming hydrogen bonds, it exhibits the highest boiling point.
