Question
Question: Rank the bond dissociation energies of the bonds indicated with the arrows. (from smallest to larges...
Rank the bond dissociation energies of the bonds indicated with the arrows. (from smallest to largest)
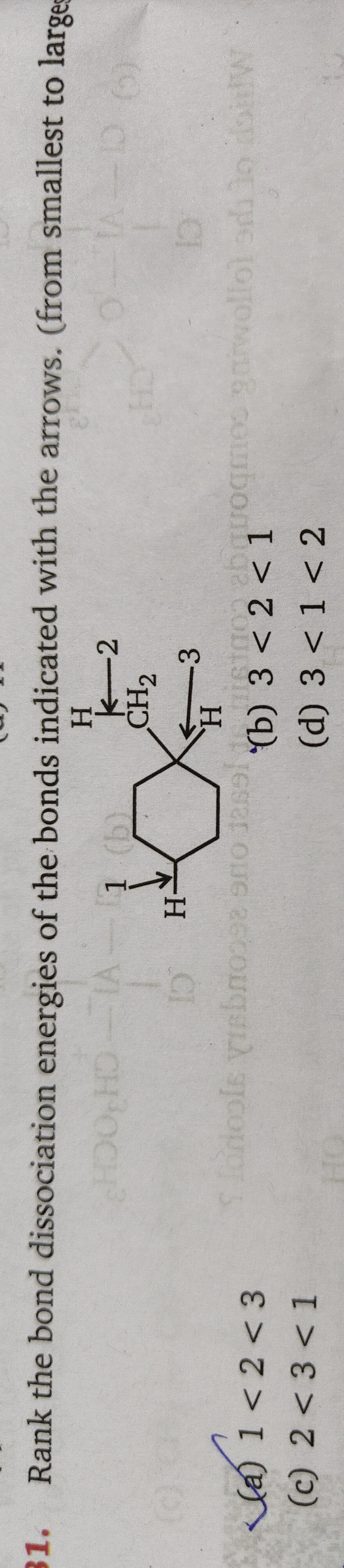
1<2<3
3 < 2 < 1
2 < 3 <1
3 < 1 < 2
3 < 2 < 1
Solution
The bond dissociation energy (BDE) of a C-H bond is inversely related to the stability of the radical formed. Tertiary radicals are more stable than secondary radicals due to hyperconjugation. Bond 3 is a tertiary C-H bond, forming a tertiary radical, hence it has the lowest BDE. Bonds 1 and 2 are secondary C-H bonds. Comparing the stability of the secondary radicals formed from bonds 1 and 2, the radical from bond 2 is more stable because its adjacent carbons provide more alpha-hydrogens (3) for hyperconjugation compared to the radical from bond 1 (2 alpha-hydrogens). Therefore, BDE(3) < BDE(2) < BDE(1).
