Question
Question: Observed the following reaction sequence and identify the correct options?...
Observed the following reaction sequence and identify the correct options?
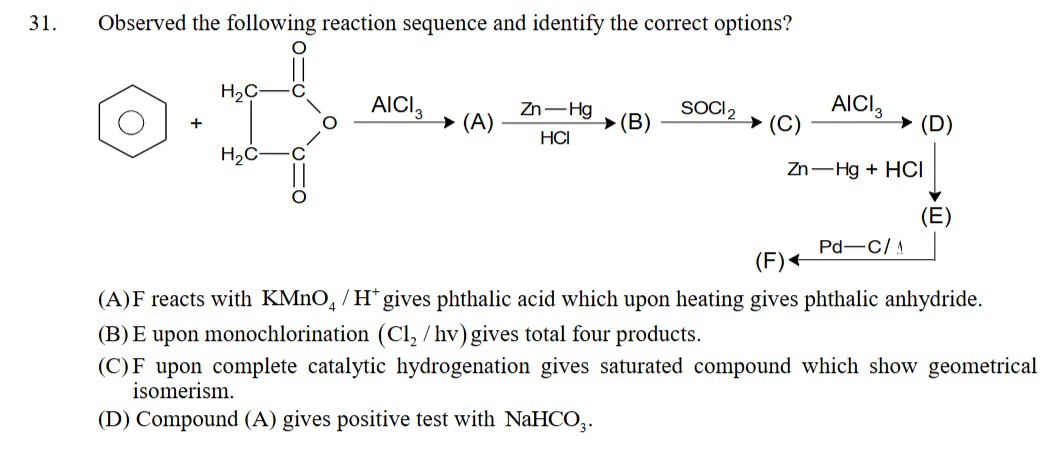
F reacts with KMnO4 / H+ gives phthalic acid which upon heating gives phthalic anhydride.
E upon monochlorination (Cl2 / hv) gives total four products.
F upon complete catalytic hydrogenation gives saturated compound which show geometrical isomerism.
Compound (A) gives positive test with NaHCO3.
A, B, C, D
Solution
- Benzene + Succinic anhydride AlCl3 (A): Friedel-Crafts acylation yields 4-oxo-4-phenylbutanoic acid (A).
- A Zn−Hg/HCl (B): Clemmensen reduction converts the ketone to a methylene group, yielding 4-phenylbutanoic acid (B).
- B SOCl2 (C): Thionyl chloride converts the carboxylic acid to an acyl chloride, yielding 4-phenylbutanoyl chloride (C).
- C AlCl3 (D): Intramolecular Friedel-Crafts acylation forms a 6-membered ring, yielding 1-tetralone (D).
- D Zn−Hg/HCl (E): Clemmensen reduction of the ketone yields tetralin (E).
- E Pd−C/Δ (F): Catalytic dehydrogenation of tetralin yields naphthalene (F).
Evaluating options: (A) Naphthalene (F) oxidation yields phthalic acid, which dehydrates to phthalic anhydride upon heating. Correct. (B) Tetralin (E) has 4 types of non-equivalent hydrogens (2 on the aromatic ring, 2 on the saturated ring - benzylic and non-benzylic), leading to 4 monochlorination products. Correct. (C) Complete hydrogenation of naphthalene (F) gives decalin, which exists as cis and trans isomers (geometrical isomerism). Correct. (D) 4-oxo-4-phenylbutanoic acid (A) has a carboxylic acid group, which reacts with NaHCO3 to give a positive test. Correct.
